Fatty acid composition of triacylglycerols
Lipids are also main components of the human diet. The consumer preference for plant-derivedoils is increasing to the detriment of animal fats. Annual plant oil production is increasing worldwide and most of it is used for human consumptionas margarines, oils and food ingredients. The triacylglycerolsare the most important components of plant seed oils. Interestingly, the physical and chemical properties of an edible oil are related to the chemical structure of the fatty acidsesterifying the glycerol (Table6.1). Properties such as melting point, colour, flavour, mouthfeel, spreadability, stability, and effects on human health are determined by the fatty acid composition of the triacylglycerols. Most efforts in developing changes in the lipid composition of plant oils have been directed to change the proportion among the fatty acids of the triacylglycerols.Common fatty acids in the commercial seed oils are lauric, myristic, palmitic, stearic, oleic, linoleic and linolenic. As is apparent, their differences occur in the length of the carbon skeleton (C12 to C20) as well as in the presence of double bonds (unsaturations). Long chain fatty acids containing two or more double bonds are named polyunsaturated fatty acids (PUFA). Different studies on the effect of dietary fatty acids consumption on human health have noticed the trend of consumers towards a reduction of saturated acids in the diet and, accordingly, an increase in unsaturated acids. Epidemiological studies have shown that intake of monounsaturated acids was associated with a low incidence of coronary artery disease (Keys et al., 1986), which has been explained by its reduction in the low density lipoproteins (LDL) levels and their oxidation (Mata et al., 1997).
| Table 6.1 Nomenclature and represntative examples of naturally occuring fatty acids | |||||
| Systematic nomenclature | m : n ΔaZbZ... | ||||
| m : carbon atoms | |||||
| n : double bonds | |||||
| superscript : position of the double bonds | |||||
a, b, ... : carbon numbered from the carboxyl end |
|||||
Z : configurationcis of the double bond |
|||||
| Examples: | |||||
Saturated |
18 : 0 | Stearic acid | |||
Unsaturated |
18 : 1Δ9Z | Oleic acid | |||
Polyunsaturated (PUFA) |
20 : 5Δ5Z,8Z,11Z,14Z,17Z | Eicosapentaenoic acid | |||
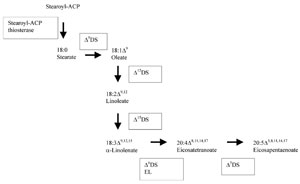
Fig. 6.2 Interconversionsof the fatty acidsindicatedin Table6.1 asexamples, Enzymes: ΔnDS: n-desaturase: EL: elongase.
Interestingly, there have been cases where saturation of the fatty acids has been the purpose of the plant genetic modification. Saturation of fatty acids determines properties such as melting temperature and viscosity that may be important to a commercial product. Margarine, for example, needsto be easily spreadable with in a range of temperatures. In addition, saturation may also be beneficial for oil stability since it is known that unsaturated acids are more readily oxidised, resulting in an increased tendency to rancidity and off odours. Finally, vegetable oils used for frying require partial saturation by hydrogenation in order to give adequate characteristics of stability and melting temperature to these oils. The chemical hydrogenation has been proven to induce also a change in the configuration of the double bonds of the fatty acids, from the naturally occurring cis to trans. The presence of the trans unsaturated fatty acids has been correlated to a risk of coronary heart disease. Therefore, plants with a high content of natural oil and with a high level of saturation have been engineered. High stearate content of the oil in a Brassica plant has been achieved by two methods. One method transformed this plant species with the antisense construct of the gene encoding the stearoyl-ACP desaturase, the enzyme that catalyses the transformation of stearic acid into oleic acid (Fig. 6.2, Δ9DS) (Knutzonet al. 1992). The silencing of the endogenous gene produced the accumulation of stearic acid up to 40% of the total fatty acids content. The second method transformed the Brassica plant with a gene encoding the stearoyl -ACP thiosterase specific for the synthesis of stearic acid (Fig. 6.2). Using this approach, the transgenic plants yielded up to 68% of this fatty acid.




