Alcohols : The Liquid Fuel
General Account
In 1980, U.S.A. commercialized the 'gasohol'. This work boosted up alcohol production in the country even from cereals. Moreover, addition of methanol (a wood alcohol) in petrol has already caught on in the U.S., with President Bush endorsing the production of 1,000,000 alternative fuel vehicles.
India is fortunate enough for having many sources of biomaterials to be used in ethanol production. The Government is facing a crisis in case of molasses. Every year, potatoes have rotten for lack of buyers. Cassava is grown on large scale in Kerala and some parts of Tamil Nadu (see Sources of wastes). A large number of distilleries are in operation and many more to be set up. Utilization of sugary and starchy materials for the production of ethanol would be a good step to cut down the oil price and meet the fuel demand in country.| Physico-chemical properties | Petrol | Ethanol |
| Freezing point (°C) | < -130 | - 117 |
| Boiling point (°C) | 35-200 | 78 |
| Energy value (MJ kg-1) | 44.0 | 27.2 |
| Density (kg I-1) | 0.74 | 0.79 |
| Flashpoint (°C) | 13 | 45 |
| Latent heat of vaporization (MJ kg-1) | 293 | 855 |
| Octane number | 80-100 | - 117 |
Ethanol is produced by chemical as well as biological routes. Through the chemical route, synthetic alcohol is produced by catalytic hydration of ethylene (C2H2) with water, using phosphoric acid at 70 atmosphere pressure and 300°C. This process involves the use of petroleum as fuel to generate high pressure and temperature for producing alcohol. Ethylene is derived from both natural and coke oven gases, and the waste gases released in refining petroleum to produce gasoline.
Biological route is an alternative way to produce alcohol. The biomass to be converted into liquid fuel can be derived from agriculture, municipal or forestry wastes (see Sources of wastes). The agricultural crops contain sugar (e.g. sugar cane, sugar beet), starch (maize, tapioca) or cellulose (cotton, species of populus) in high amount. Dr. T.K. Ghosh and coworkers of IIT, New Delhi are making effort to use lignocellulosic materials for alcohol production in addition to starch based substrates.
Following are the types of substrates used for alcohol production :
Sugary Materials: Examples of sugary materials are sugarcane and its by products/wastes (molasses, bagasse) and sugar beet, tapioca, sweet potatoes, fruit juice, sweet sorghum, etc. Sugar cane molasses is largely being used in many country for alcohol production.
Starchy Materials:Starchy materials used in ethanol production are tapioca, maize, wheat, barley, oat, sorghum, rice and potatoes. But tapioca and corns are the two major substrates of the interest (see Sources of wastes). It has been estimated that 11.7 kg of corn starch can be converted into about 7 liters of ethanol (Chahaf and Overend, 1982).
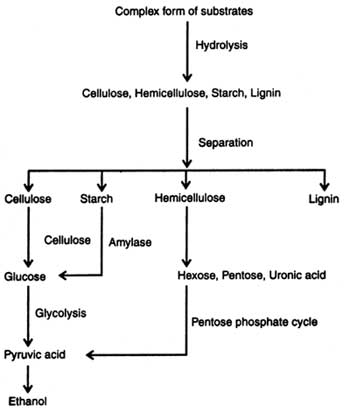
Lignocellulosic Materials: The sources of cellulosic and lignocellulosic materials are the agricultural wastes and wood. However, yield of ethanol from lignocellulose is low because of lack of suitable technology and failure of conversion of pentoses into ethanol. On the basis of technology available today about 409 liters of ethanol can be produced from one tonne of lignocelluloses (Chahal and Overend, 1982). Structure of cellulose, hemicellulose and lignin is given in Figs. 19.1-19.4 and anaerobic hydrolysis of these is discussed in the preceding section. Production of ethanol from lignocelluloses follows the following steps: (i) hydrolysis, (ii) fermentation, and (iii) recovery.
Hydrolysis of lignocelluloses are done to release the monomer sugars. These materials are hydrolyzed by acids and/or enzymes. Acid hydrolysis is performed by using dilute sulphuric acid (0.1 -0.2%) through layers of saw dust or wood chips under pressure and a high temperature (180-121°C). Sugars produced during hydrolysis are destroyed by acid. Therefore, it must be quickly removed. Acid portion should be neutralized with lime before the solution is fermented (Chahal and Overend, 1982). The other chemicals used in hydrolysis are hydrochloric acid, sodium hydroxide, ammonia, etc. Substrate hydrolysis coupled with high pressure and temperature for varying times, depending on nature of substrate, has shown a good result.
Enzymatic hydrolysis of cellulosic and lignocellulosic materials is performed by using the respective enzymes i.e. cellulases, hemicellulases, pectinases, lignases, etc. These enzymes are produced on a large scale from microorganisms (see Enzyme Technology). Enzymatic hydrolysis of cellulose, hemi-cellulose and lignin is discussed elsewhere (see Enzymatic digestion).
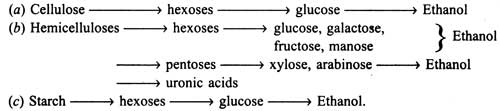
Upon hydrolysis of carbohydrates of plant materials the following sugars are liberated in the solution which in turn are fermented into ethanol :
Effect of Substrate Composition on Hydrolysis
Composition of substrates directly governs the hydrolysis, such as insolubility, high crystallinity and lignin coating (over the cellulose microfibrils in cell wall).
Crystallinity of cellulose directly affects its hydrolysis by cellulases. Cellulases will readily degrade the amorphous portion of cellulose, but not the crystalline portion, until the cellulase complex is rich in Cx enzyme. Lignin hinders substrate hydrolysis. As the amount of lignin is high in substrate, the yield of ethanol would be low. Treatment of substrate with alkali (2% NaOH at 70°C for 90 minutes, washed and sterilized) would be more effective than without treatment.
Moreover, steam explosion (i.e. steam treatment at 190°C) of lignocellulosic materials is another pre-treatment which has proved to be the best for hydrolysis (Chahal and Overend, 1982).
The soluble hexoses and pentoses obtained in solution after hydrolysis of sugary-starchy-cellulose are subjected to anaerobic fermentation by using bacteria, yeast and filamentatous fungi (see Alcohols). Theoretically, maximum conversion of glucose to ethanol is 51 per cent by weight. Yeast ferments glucose to 10-20 per cent ethanol. An outline of ethanol production is given in Fig. 20.1. The micro-organisms associated with fermentation utilize various metabolic process, depending on substrates i.e. glucose, xylose, etc. (see Metabolic pathways in microorganisms).
Recovery of Ethanol
When fermentation is over, the microbial mass (cells, mycelia and conidia) is separated from the fluid. The fluid contains the mixture of ethanol, water and small amount of alcohols and ether. Ethanol is recovered by distillation process i.e. vaporization of ethanol/water mixture.