Symbiotic N2 Fixation
The classical examples of symbiotic association developed by Rhizobium sp. are found in about 13,000 or more leguminous plants, both cultivated and non-cultivated herbs, shrub and trees. Among leguminosae, the largest number of plants is in papilionoidae. Moreover, species of Anabaena, Nostoc, Tolypothrix, etc. develop symbiotic association with fungi (symbiotic structure is lichen), bryophytes, pteridophytes, gymnosperms and angiosperms and fulfill the requirement of nitrogen deficiency (Table 11.1). However, actinorrhizic nodules are developed by Frankia, a member of Actinomycetes, on roots of about 170 species of woody dicot non-leguminous plants from 15 genera belonging to temperate trees like Alnus, Myrica, Casuarina, etc. (Goodfellow and Williams, 1983). Nodules; are of two types: (i) Alnus type where nodules show dichotomous branching to form a corraloid root, and (ii) Myrica/Casurina type in which case the apex of each nodule produces a normal but negative geotropic root. The function of nodules is to facilitate gas diffusion to the nitrogen fixing endophyte in the nodule under low O2 tension (Becking, 1982).
Table 11.1. Symbiotic microorganisms.
| Microorganisms | Symbiotic structures | Micro-/macro-symbionts | |
| 1. | Bacteria* | ||
| Azotobacter paspali | No special structure develops, intimately associated with roots | Paspalum notatum, roots of corn,wheat, sorghum, Digitaria decombense | |
| Azosipiillum amazonense | |||
| A. brasilense | |||
| A. ligoferum | |||
| Beijerinckia sp. | |||
| Derxia sp. | |||
| Rhizobium sp. | Root nodules | Leguminous plants, and non-leguminous plant (e.g. Trema canabaena) | |
| 2. | Actinomycetes Frankia sp | Root nodules | Non-leguminous angiosperms eg. Alnus, Casuarina, Myrica, Discaria |
| 3. | Cyanobacteria | ||
| Anabaena, Nostoc and Tolypothrix | Lichens (Collema, Peltigera, Usnia) | Fungi: Ascomycetous and basidiomycetous fungi |
|
| Nostoc sp. | — | Bryophytes: Anthoceros, Blasia | |
| Anabaena azollae | — | Pteridophytes : Azolla | |
| A. cycadae | Coralloid roots | Gymnosperms : Cycas | |
| Nostoc | — | Angiosperm : Gunnera |
Elkan (1985) grouped the nodule forming bacteria into the following two:
| Genus I: | Rhizobium - it consists of the fast growing and the flagellated strains. The species are : (i) R. leguminosarum biovars. trifoli : host - Trifolium, and R. leguminosarum biovars . phaseoli : host - Phaseolus (ii) R. meliloti : host - Lotus. |
| Genus II. | Bradyrhizobium - it contains the slow growing or sub-polar flagellated strains. (i) B. japonicum : host - Glycine (ii) Brady rhizobium sp. : host - Cicer, Vigna, Cajanus. |
Establishment of Symbiosis
Establishment of Rhizobium inside the host root and development of nodules are a complex process which follow many events such as recognition and infection of host root, differentiation of nodules, proliferation of bacteria and conversion into bacteroids in nodules. These steps are briefly described as below :
Table 11.2. Species of Rhizobium and cross inoculation groups of host plants.
| Rhizobium sp. | Host genera | Cross inoculation |
| R. japonicum | Glycine | Soybean groups |
| R. leguminosarum | Pisum, Lathyrus, Vicia, Lens | Pea group |
| R. lupini | Lupinus, Ornithopus | Lupin group |
| R. meliloti | Melilotus, Medicago, Trigonella | Alfalfa group |
| R. phaseolus | Phaseolus | Bean group |
| R. trifolii | Trifolium | Clover group |
| Other species of Rhizobium | A rack is, Crotalaria, Vigna, Pueraria | Cowpea group |
Host plant secretes exudates in rhizosphere ; subsequently compatible strains of rhizobia are stimulated over the other microbes in soil. Root exudates contain growth stimulating substances like biotin, thiamine, amino acids, etc. Bacteria grow near the root surrounded by mucigel. Mucigel denotes microbial cells and their products together with associated microbial cells and mucilages, organic and inorganic matter in the rhizosphere of root region. The initial host response is observed as root curling which is known as Shepherd's Cook (Fig. 11.6.A). Root curling takes place due to secretion by Rhizobium of a curling factor which includes cytokinin, polymixin B, etc. (Dart, 1974).
Infection of root hairs
Rhizobial aggregates have been observed at distinct sites on curled root hairs (Fig. 11.6 A II). Nutman (1956) has suggested that the infection thread is formed by a process of invagination of the hair cell walls in the region of curiing. This process of invagination is being repeated at each cell penetrated by infection thread. Root hair wall invaginates until it develops a tube like structure.
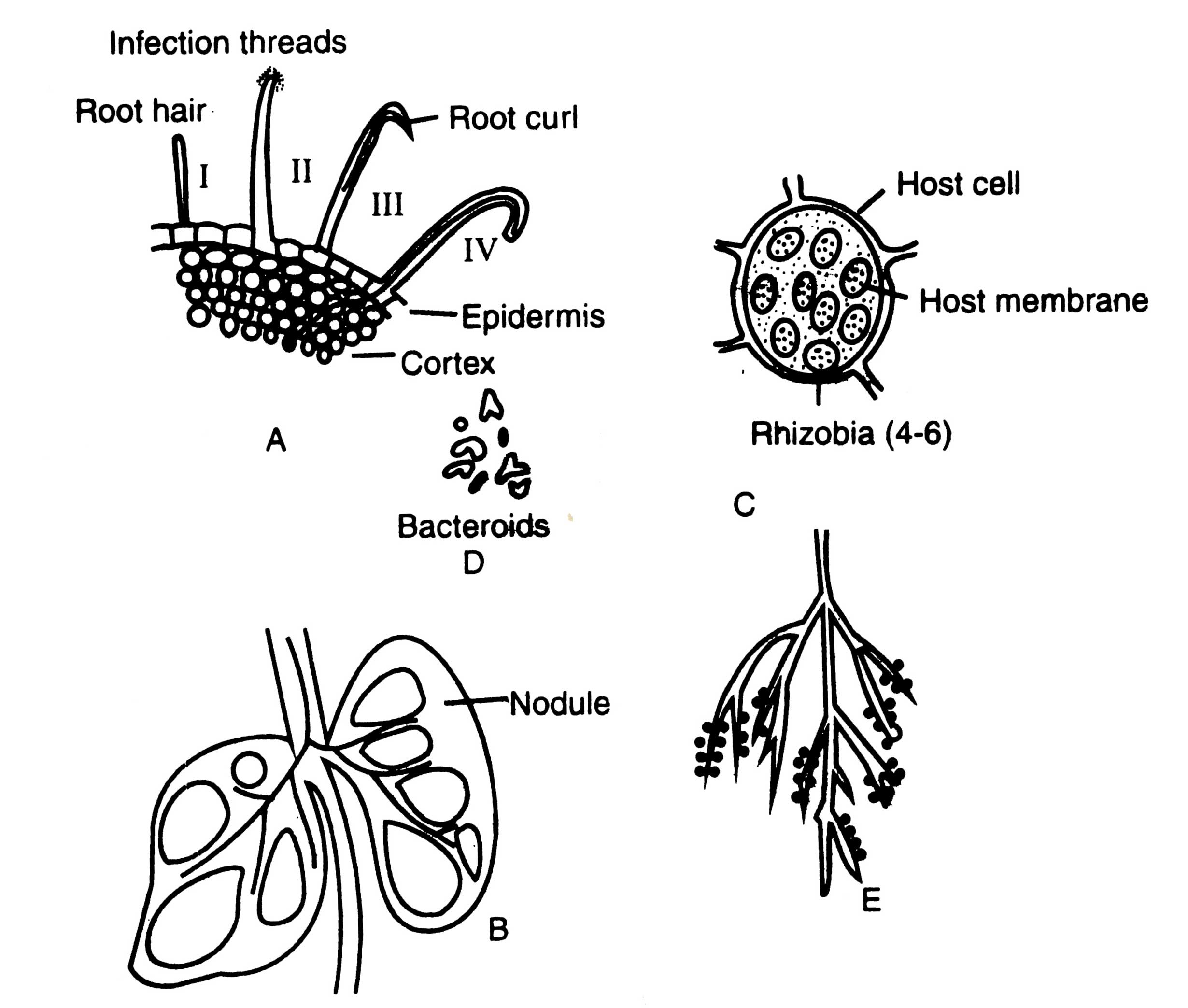
Fig. 11.6. Root nodule formation in a leguminous plant. A-transverse section of a root showing root hairs and its invasion by infection threads of Rhizobium sp.; B-vertical section through nodules; C-a cell of the infected host filled with Hiizobia; D-various shape of bacteroids; E-root system of a leguminous plant showing numerous nodules.
Deformed hairs are penetrated in the first stage of infection (Fig. 11.6 A III). Not all, only a few proportion (about 5%) of young root hairs develop nodules. Following the penetration, a hypha like infection thread is formed. The infection threads resemble to invading fungal hyphae. It is a unique structure which contains a cellulose sheath deposited by the host cell enclosing a strand of hemicelluiosic substance in which the bacteria are embeded (Schaede, 1940). If the population of rhizobia is even in the infected tubes, it can be observed under light microscope also. The infection thread grows towards root cortex and ramify throughout the central part of cortex (Fig. 11.6 A IV). It is noteworthy that plasmoptysed hairs have not been observed to form infection threads, although they can be invaded by bacteria. Plasmoptysis may be an important mechanism of root exudation (Nutman, 1965).
Nodule formation
As the infection thread continues to grow through the root tissue, inner cortical cells are stimulated by bacteria through growth hormone to divide and form an organized mass of infected plant tissue which protrudes from the root surface as a visible nodule (Fig. 11.6B). Rhizobia are released from the infected threads, within some of the cortical cells of nodule and multiply thereby rapid cell division, and ultimately occupy the major central portion of root nodule. The peculiar feature of cells of central nodule is that it contains tetraploid chromosome number. The chromosome doubling of nodule cells may be attributed to secretion of stimulating chemicals by rhizobia.
Many plant and bacterial genes express in an sequential manner during nodule development and maintenance in legumes. Legochi and Verma (1980 studied the Rhizobium - legume symbiosis. They found the expression of plant genes and production of nodule specific protein during certain stages of nodules. They termed these proteins as 'nodulins'. On the basis of functions, nodulins have been categorized into three (i) nodule structure maintaining proteins, (ii) bacteroid function (N2 - fixation) supporting proteins and (iii) proteins (enzymes) expressed in specific nitrogen assimilation and carbon metabolism (Fuller et al, 1983). Moreover, on the basis of structural resemblance nodulins are divided into two : C-nodulins and S- nodulins. C-nodulins (common nodulins) are the proteins common to all nodules, while S-nodulins are referred as species specific nodulins and not found commonly in all species.




