Molecular Farming from Transgenic Plants
Recent progress in areas of transgenic plants have attracted the attention of the scientists. The plants are being looked upon as potential bioreactors or biofactories for the production of immunotherapeutic molecules
i.e. molecular farming. Transgenic material in the form of seed or fruit can be easily scored and transported from one place to another without fear for its degradation or damage. (Besides, transgenic plants capable of producing several different products can be created at any time by crossing the plants that produce different product^ (Sharma
et al, 1999).
Immunotherapeutic drugs
For the first time Hiatt
et al. (1989) produced antibodies in plants which could produce positive immunization. But the first report on production of edible vaccine appeared in 1990 in the form of a patent application. In 1992, C.J. Arntzen and co-workers expressed hepatitis B surface antigen in tobacco to produce immunologically active ingredients via genetic engineering of plants. During 1980s, great effort has been made to transform plant by foreign genes. Various foreign proteins including serum albumin, human a-interferon, human erythropoietin, and murine IgG and IgA immunoglobulins have been successfully expressed in plants. Antigens and antibodies expressed in plants can be administered orally as any edible part of the plants, or by parental route after purification from the plants. The edible part of the plant to be used as vaccine is fed as raw material to experimental animal or humans. After cooking they are denatured. Therefore, for the production of edible vaccines or antibodies, it is desired to select a plant whose products are consumed raw to avoid degradation during cooking. The plants of choice are tomato, banana and cucumber (Sharma
et al, 1999).
In 1999, the Indian scientists at ICGEB, New Delhi have successfully produced transgenic maize, tobacco, rice, etc. capable of producing interferon gamma (INF-γ).
(i) Edible vaccines. The plants are capable of producing vaccines in large quantities at low cost but the purification may require more cost. Therefore, attention has been paid to produce such antigens that stimulate mucosal immune system to produce secretary IgA (S-IgA) at mucosal surface such as gut and respiratory epithelia because of their effectiveness on sites as most of the pathogens invade these regions. For example, bacteria and viruses are transmitted via contaminated food or water and cause diseases such as diarrhoea, whooping cough, etc. In 1990, the first report of the production of edible vaccine (a surface protein from
Streptococcus) in tobacco at 0.02 per cent of total leaf protein level was published in the form of a patent application under the International Patent Cooperation Treaty (Mason and Arntzen, 1995). Thereafter, expression of a number of antigens in plants was successfully made and reported (Table 9.9).
Table 9.9. Antigens produced in transgenic plants.
| |
Plants |
| Hepatitis B surface antigen |
Tobacco |
| Rabies virus glycoprotein |
Tomato |
| Norwalk virus capsid protein |
Tobacco |
| E.coli heat-labile enterotoxin b-subunit |
Potato |
| Cholera toxin b-subunit |
Potato, tobacco |
| Mouse glutamate dehydrogenase |
Potato |
| VP1 protein of Foot & mouth disease virus |
Arabdiopsis |
| Insulin |
Potato |
| Glycoprotein of swine-transmissible gastroenteritis coronavirus |
Arabdiopsis |
Acute watering diarrhoea is caused by enteroxigenic
Escherichia coli and
Vibrio cholerae thatcolonize the small intestine and produce enterotoxin. Cholera toxin (CT) is very similar to
E.coli toxin. The CT has two subunits, A and B. Attempt was made to produce edible vaccine by expressing heat-labile enterotoxin (CT-B) in tobacco and potato.
A tobacco plant was produced that expressed CT-A or CT-B subunits of the toxin CT-A. CT-A produced in plant was not cleaved into CT-Al and CT-A2 subunits which generally happens in epithelial cells. Similarly, CT-B subunit when expressed in potato was processed in natural way, the pentameric form (the naturally occurring form) being the abundant form. Even after boiling transgenic potato tubers till they became soft, about 50 per cent of the CT-B was present in the pentameric GM1 ganglioside-binding form (Arakawa
et al, 1997).
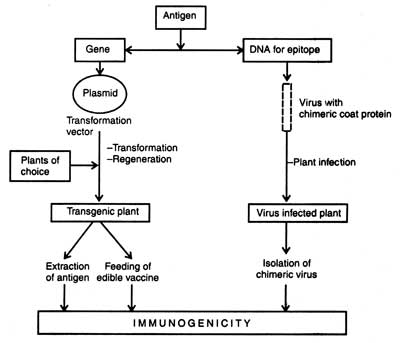
Fig. 9.8. Outline of production of candidate vaccine through transgenic plants (based on Sharma et al., 1999)
Similarly, a rabies virus coat glycoprotein gene has been expressed in tomato plants. Orally administered protein elicited protective immunity in animals. It was hoped that after further effort an edible oral vaccine against rabies can be developed. The value of vaccine can be improved by providing other adjuvants which either enhance the immunogenic potential or reduce degradation of the active ingredients by the microorganism of gut (McGarvey
et al, 1997).
In transgenic tobacco plants the hepatitis B surface antigen (HBsIg) accumulates to 0.01 per cent of soluble protein level. The HBsIg was recovered in virus like particles of 22 nm diameter (similar to yeast-derived HBsIg-based vaccine) which is known to be a prerequisite for better immunogenicity. A crude extract from plant was used for immunization in mice. Immune response included all IgG subclasses and IgM against hepatitis B.
One of the alternative strategies of producing a plant-based vaccine is to infect the plants with recombinant virus carrying the desired antigen that is fused to viral coat protein.
The infected plants have been reported to produce the desired fusion protein in large amounts in a short duration. The technique involved either placing the gene downstream a subgenomic promoter, or fusing the gene with capsid protein that coats the virus (Fig. 9.8; Table 9.10).
Table 9.10. Transient production of antigens in plants after infection with plant viruses expressing a recombinant gene.
| Protein |
Plant |
Carrier |
| Influenza antigen |
Tobacco |
Tobacco mosaic virus |
| Murine zona pellucida antigen |
Tobacco |
Tobacco mosaic virus |
| Rabies antigen |
Spinach |
Alfalfa mosaic virus |
| HIV-1 antigen |
Tobacco |
Alafalfa mosaic virus |
| Mink enteritis virus antigen |
Black eyed bean |
Cowpea mosaic virus |
| Colon cancer antigen |
Tobacco |
Tobacco mosaic virus |
Source : Sharma
et al (1999).
(ii) Edible antibodies. Transgenic plants are being looked upon as a source of antibodies also which can provide passive immunization by direct application. They provide as a tool for drug targeting. Gene technology has provided impetus to the utility of antibodies. The genes coding for both light and heavy chains have been expressed. Moreover, the modified genes capable of expressing Fab fragments (assembled light chain and shortened heavy chains) or scFV (single peptide chain where variable domains of heavy and light chains are covalently linked by a short flexible peptide) have also been expressed in bacteria and mammalian cells (Fig. 9.9; Table 9.11). Murine antibodies have been humanized by changing the constant and framework domains. Besides, the recent technology involving PCR and phage display allow cloning and screening of antibodies (Sharma
et al, 1999).
Table 9.11. Antibodies and antibody fragments produced in transgenic plants.
| Antibody |
Antigen |
Plant |
| IgG (k) |
Transition stage analogy |
Tobacco |
| IgM (l) |
NP (4-hydroxy-3-nitrophenyl)
acetyl hapten |
Tobacco |
| Single domain (dAb) |
Substance P |
Tobacco |
| Single chain Fv |
Phytochrome |
Tobacco |
| IgG |
Glycoprotein B of herpes
simplex virus |
Soybean |
| Fab : IgG (k) |
Fungal cutinase |
Tobacco |
IgG (k) and SIgG/A
hybrid |
Streptococcus mutans |
Tobacco |
| Single chain Fv |
Abscisic acid |
Tobacco |
Source : Many research papers.
Ma
et al (1995) successfully produced multimeric secretary IgA (SIgA) molecules in plants which represent the predominant form of immunoglobulin in mucosal secretions. SIgA contained light and heavy chains, and domainized by a J-chain. The chains were protected by a fourth polypeptide. Thus, four transgenic tobacco plants were produced by genetic engineering which produced a murine monoclonal antibody like K-chains, hybrid IgA-G antibody heavy chain, murine J-chain and rabbit secretary component. A series of sexual crosses were carried out to allow expression of all the four proteins simultaneously. The progenies produced a functional sectary immunoglobulin very efficiently. This demonstrates the potential of plants in assembly of antibodies, and flexibility of system (Fig. 9.9).
A hybrid monoclonal antibody (IgA/G) having constant regions of IgG and IgA fused, has been used successfully against human dental carries caused by the bacterium,
Streptococcus mutans.
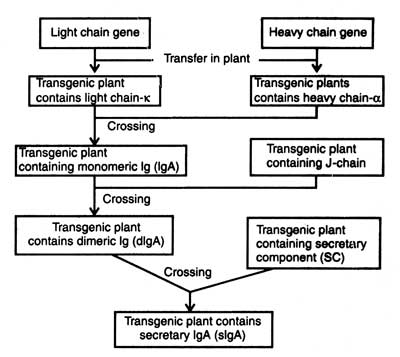
Fig. 9.9. Outline for production of secretory antibody in plants.
The secretary antibody generated SIgA/G in transgenic tobacco and the original mouse IgG was compared. It is interesting to know that both had similar binding affinity to surface adhesion protein of
S. mutans. SIgA/G survived for 3 days in the oral cavity, whereas IgG survived only for one day. The plant antibody provided protection against the colonization of
S. mutans for at least four months (Ma
et al, 1998).
(iii)Edible interferon. Scientists at ICGEB have successfully produced transgenic tobacco and maize plants that secrete human interferon (IFN-y). It was produced by transformation of nucleus and chloroplast through particle bombardment method. It has been found that interferon was 10-15 fold greater in chloroplast-transformed plants than nucleus-transformed plants.






