Electrophoresis
Electrophoresis is defined as the separation (migration) of charged particles through a solution or gel, under the influence of an electrical field. The rate of movement of particle depends on the following factors.-
The charge of the particle
-
Applied electric field
-
Temperature
-
Nature of the suspended medium.
What is Gel Electrophoresis?
Gel electrophoresis is a method that separates macromolecules—either nucleic acids or proteins—on the basis of size, electric charge, and other physical properties. A gel is a colloid in a solid form. The term electrophoresis describes the migration of charged particles under the influence of an electric field. “Electro” refers to the energy of electricity. “Phoresis,” from the Greek verb phoros, means “to carry across.” Thus, gel electrophoresis refers to the technique in which molecules are forced across a span of gel, motivated by an electrical current. Activated electrodes at either end of the gel provide the driving force. A molecule’s properties determine how rapidly an electric field can move the molecule through a gelatinous medium.
Many important biological molecules such as amino acids, peptides, proteins, nucleotides, and nucleic acids, possess ionizable groups and, therefore, at any given pH, exist in solution as electrically charged species, either as cations (+) or anions (–). Depending on the nature of the net charge, the charged particles will migrate to either the cathode or the anode.
How does this Technique Work?
Gel electrophoresis is a technique used for the separation of nucleic acids and proteins. Separation of large (macro) molecules depends upon 2 forces: charge and mass. When a biological sample, such as proteins or DNA, is mixed in a buffer solution and applied to a gel, these 2 forces act together. The electrical current from one electrode repels the molecules, while the other electrode simultaneously attracts the molecules. The frictional force of the gel material acts as a “molecular sieve,” separating the molecules by size. During electrophoresis, macromolecules are forced to move through the pores when the electrical current is applied. Their rate of migration through the electric field depends on the strength of the field, size, and shape of the molecules, relative hydrophobicity of the samples, and on the ionic strength and temperature of the buffer in which the molecules are moving. After staining, the separated macromolecules in each lane can be seen in a series of bands spread from one end of the gel to the other.
Agarose
There are 2 basic types of materials used to make gels: agarose and polyacrylamide. Agarose is a natural colloid extracted from seaweed. It is very fragile and easily destroyed by handling. Agarose gels have very large “pore” size and are used primarily to separate very large molecules, with a molecular mass greater than 200 kdal. Agarose gels can be processed faster than polyacrylamide gels, but their resolution is inferior. That is, the bands formed in the agarose gels are fuzzy and spread far apart. This is a result of pore size and cannot be controlled.
Agarose is a linear polysaccharide (average molecular mass about 12,000) made up of the basic repeat unit agarobiose, which composes alternating units of galactose and 3,6-anhydrogalactose. Agarose is usually used at concentrations between 1% and 3%.
Agarose gels are formed by suspending dry agarose in an aqueous buffer, then boiling the mixture until a clear solution forms. This is poured and allowed to cool to room temperature to form a rigid gel.
Polyacrylamide
There are 2 basic types of materials used to make gels: agarose and polyacrylamide. The polyacrylamide gel electrophoresis (PAGE) technique was introduced by Raymond and Weintraub (1959). Polyacrylamide is the same material that is used for skin electrodes and in soft contact lenses. Polyacrylamide gel may be prepared so as to provide a wide variety of electrophoretic conditions. The pore size of the gel may be varied to produce different molecular seiving effects for separating proteins of different sizes. In this way, the percentage of polyacrylamide can be controlled in a given gel. By controlling the percentage (from 3% to 30%), precise pore sizes can be obtained, usually from 5 to 2000 kdal. This is the ideal range for gene sequencing, protein, polypeptide, and enzyme analysis. Polyacrylamide gels can be cast in a single percentage or with varying gradients. Gradient gels provide a continuous decrease in pore size from the top to the bottom of the gel, resulting in thin bands. Because of this banding effect, detailed genetic and molecular analysis can be performed on gradient polyacrylamide gels. Polyacrylamide gels offer greater flexibility and more sharply defined banding than agarose gels.
Mobility of a molecule = |
(applied voltage) × (net charge of the molecule) / friction of the molecule (in the electrical field) | |
v (velocity) = |
E (voltage) × q (charge)/f (frictional coefficient). |
Polyacrylamide Gel Electrophoresis
Polyacrylamide is the solid support for electrophoresis when polypeptides, RNA, or DNA fragments are analyzed. Acrylamide plus N,N’-methylene-bis-acrylamide in a given percentage and ratio are polymerized in the presence of ammonium persulfate and TEMED (N,N,N’,N’-tetra-methyl-ethylene-diamine) as catalysts.
Safety and Practical Points
- Acrylamide and bis-acrylamide are toxic as long as they are not polymerized.
- Buffer (usually Tris) and other ingredients (detergents) are mixed with acrylamide before polymerization.
- Degassing of acrylamide solution is necessary before pouring the gel because O2 is a strong inhibitor of the polymerization reaction.
- Under nondenaturing conditions.
- Under denaturing conditions.
- Isoelectric focusing.
These techniques are used to analyze certain properties of a protein such as: isoelectric point, composition of a protein fraction or complex, purity of a protein fraction, and size of a protein.
We will concentrate on denaturing polyacrylamide gel electrophoresis in the presence of sodium dodecylsulfate (SDS-PAGE) and a reducing agent (DTT, or dithioerithritol, DTE).
The protein is denatured by boiling in “sample buffer,” which contains: - Buffer pH 6.8 (Tris-HCl).
- SDS.
- Glycerol.
- DTT or DTE.
- Bromophenol blue (tracking dye).
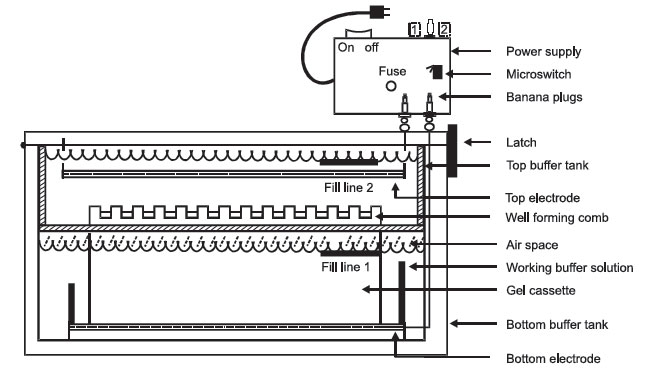 |
FIGURE 1 Electrophoresis. |
Discontinuous Polyacrylamide Gel Electrophoresis
This type of polyacrylamide gel consists of 2 parts:
- The larger running (resolving) gel
- The shorter upper stacking gel.
The running gel has a higher percentage (usually 10%–15%) of acrylamide and a Tris-HCl buffer of pH 8.8.
The stacking gel usually contains 5% acrylamide and a Tris-HCl buffer of pH 6.8.
The buffer used in SDS-PAGE is Tris-glycine with a pH of about 8.3.
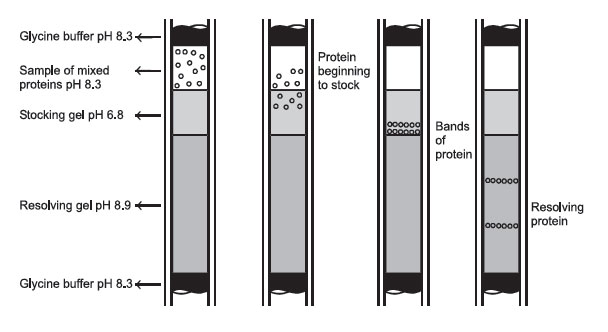 |
FIGURE 2 Protein passage through a disc-gel electrophoresis system. |
Determination of the Molecular Weight of a Polypeptide by SDS-PAGE
Since all polypeptides are wrapped with SDS and thus are strongly negatively charged, they migrate through the running gel according to their size (small polypeptides migrate faster than large ones!).
There is a linear relationship between the log of the molecular weight of the polypeptide and its migration during SDS-PAGE.
Standard polypeptides have to be run on the same gel and a curve of their migration versus the log of their molecular weight has to be generated.




