Use of Brain Cytosolic Extracts for Studying Actin-Based Motility of Listeria monocytogenes
Cell motility is essential for numerous biological events. Unicellular organisms, for instance, use directed movement to find and ingest food. In multicellular organisms, cell motility is required for the morphogenetic movements that accompany embryogenesis, fibroblast migration during wound healing, and the chemotactic movement of immune cells during an immune response. Cell motility is characterised by the formation of cellular extensions that, depending on their morphology and cellular context, are called lamellipodia, ruffles, or filopodia. In recent decades many cell biological, biochemical, and biophysical studies have established that the formation of these structures depends on the activity of the actin cytoskeleton and its associated proteins. More specifically, the assembly of a network of actin filaments at the leading edge of motile cells provides the propulsive force for the extension of these structures (see Small et al., 2002). Despite much effort, however, the complexity of cell motility has precluded the detailed analysis of the molecular mechanisms and components that govern this process.Since the mid-1980s, much work focused on the intracellular actin-based motility of the gram-positive bacterium Listeria monocytogenes. Listeria can induce its own uptake by phagocytic and nonphagocytic cells and, once free in the cytoplasm, recruits host cell cytoskeletal components, which are then rearranged into phase-dense actin tails. The assembly of actin monomers at the actin filament (+) ends abutting the bacterial surface provides the propulsive force that allows Listeria to move within the infected cells and spread to adjacent cells while avoiding exposure to the host's humoral immune system. As these bacteria imitate the protrusive behaviour of lamellipodial edges, Listeria motility is considered a simplified model system for actin filament dynamics during cell motility (Cossart and Bierne, 2001; Frischknecht and Way, 2001). As one approach towards defining the molecular basis of bacterial motility, we and others have developed simple in vitro systems that support actin-based Listeria motility based on Xenopus, platelets, and mouse brain extracts (Theriot et al., 1994; Marchand et al., 1995; Laurent and Carlier, 1998; Laurent et al., 1999; May et al., 1999). These cell-free systems in combination with bacterial genetics and cell biological studies have been essential for the characterisation of two key regulators of actin cytoskeleton dynamics: Ena/VASP proteins and the Arp2/3 complex (Pistor et al., 1995, 2000; Smith et al., 1996; Niebuhr et al., 1997; May et al., 1999; Skoble et al., 2000, 2001; Geese et al., 2002). They also provided the basis for further development of in vitro motility systems that culminated in the reconstruction of bacterial motility using a limited set of purified proteins (Loisel et al., 1999). This article describes procedures for the preparation and use of mouse brain extracts for studying Listeria motility.
II. MATERIALS AND INSTRUMENTATION
Calcium chloride (Cat. No. 102378), magnesium chloride hexahydrate (Cat. No. 105832), potassium chloride (Cat. No. 4936), HEPES (Cat. No. 10110), sucrose (Cat. No. 1.07654), sodium chloride (Cat. No. 1.06404), disodium hydrogen phosphate dihydrate (Cat. No. 1.06580), sodium dihydrogen phosphate hydrate (Cat. No. 6346), and paraffin (Cat. No. 1.07160) are from Merck. EGTA (Cat. No. E-3889), methylcellulose (Cat. No. M-0555), ATP (Cat. No. A-2383), erythromycin (Cat. No. E-6376), and lanoline (Cat. No. L-7387) are from Sigma. Chymostatin (Cat. No. 17158), leupeptin (Cat. No. 51867), pepstatin (Cat. No. 52682), PEFABLOCK (Cat. No. 31682), aprotinin (Cat. No. 13178), and creatine kinase (Cat. No. 127566) are from Boehringer-Ingelheim. Dithiothreitol (DTT, Cat. No. 43815), Tris-HCL (Cat. No. 93363), and glycerol (Cat. No. 49770) are from Fluka. Cell-Tak (Cat. No. 354241) is from BD Biosciences. Creatine phosphate (Cat. No. 621714) is from Roche. Vaseline (Cat. No. 16415) is from Riedel-de Haen. Brain heart infusion (BHI) culture medium and Bacto agar are from Difco Laboratories. Rhodamine-labelled actin (Cat. No. AR05) is from Cytoskeleton. A glass homogeniser equipped with a Teflon piston, glass slides (76 × 26 mm), glass coverslips (22 × 22mm), forceps, scissors, and razor blades are from local suppliers. Bacterial motility is observed using an Axiovert 135TV microscope (Zeiss) equipped with a Plan-Apochromat 100×/1.4 NA oil immersion objective. Images can be acquired with a cooled, back-illuminated CCD camera (TE/CCD-1000 TKB; Roper Scientific) driven by IPLab Spectrum software (Scanalitics).
III. PROCEDURES
A. Explantation of Mouse Brains
Solution
Phosphate-buffered saline (PBS): 130mM NaCl, 1.5mM NaH2PO4·H2O, and 4mM Na2HPO4·2H2O, pH 7.4. For 1 litre, weigh out 7.65g NaCl, 0.21g NaH2PO4·H2O, and 0.72 g Na2HPO4·2H2O. Dissolve in 900 ml H2O, adjust pH to 7.4 with 1N NaOH, and bring volume to 1 litre. Store at 4°C.
Steps
- Kill mice using CO2 or by cervical dislocation.
- Remove skin and fur from the head using thintipped scissors and discard.
- Cut the skull bone by sliding the scissors along the sagittal suture (Fig. 1a, dashed red line).
- Cut the frontal, parietal, and interparietal bones along their sutures and remove them (Fig. 1a, dashed green line).
- Gently pinch the surface of the brain to lift the meninges up and gently ease the brain out of the skull.
- Put explanted brains in ice-cold PBS.
- Wash brains three times in ice-cold PBS to remove tissue debris and blood residues.
- Proceed to Section III,B or flash freeze the brains in liquid nitrogen. Store frozen brains at -80°C.
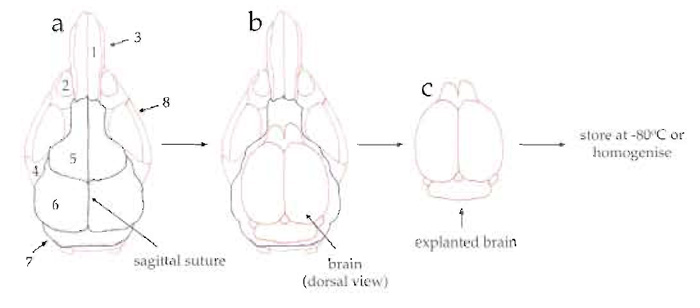 |
| FIGURE 1 Diagram of the explantation of mouse brains. (a) Dorsal view of mouse skull showing the nasal
bone (1), eye socket (2), nasal process of incisive bone (3), zygomatic process (4), frontal bone (5), parietal bone (6), interparietal bone (7), and zygomatic bone (8). (b) Dorsal view of skull after cutting (along the dashed green and red lines) and removing the frontal, parietal, and interparietal bones. (c) Dorsal view of explanted brain. |
B. Preparation of Cytosolic Extracts
Solutions
- Homogenisation buffer (HB): 20mM HEPES, pH 7.5, 100mm KCl, 1 mM MgCl2·H2O, 1 mM EGTA, and 0.2mM CaCl2. For 1 litre, weigh out 4.76g HEPES, 7.45g KCl, 0.2g MgCl2·H2O, 0.38g EGTA, and 0.02g MgCl2. Dissolve in 900ml H2O, adjust pH to 7.5 with 1N NaOH, and bring volume to 1 litre. Store at 4°C.
- Protease inhibitors: 20mg/ml chymostatin, ling/ ml leupeptin, 1mg/ml pepstatin, 167mM Pefabloc, and 10mg/m! aprotinin. To prepare stock solutions, dissolve 1mg chymostatin in 50 µl dimethyl sulfoxide, 0.5mg leupeptin in 500µl H2O, 0.5mg pepstatin in 500µl methanol, 20mg Pefabloc in 500µl H2O, and 0.5mg aprotinin in 50µl H2O. Aliquot and store at -20°C.
- 0.1M ATP: For 50ml, weigh out 2.75g ATP. Dissolve in H2O, aliquot, and store at -20°C.
- 0.1M DTT: For 50ml, weigh out 0.77g DTT. Dissolve in H2O, aliquot, and store at -20°C.
- 2M sucrose: For 20ml, weigh out 13.69 g sucrose. Dissolve in H2O, aliquot, and store at -20°C.
- Homogenisation buffer supplemented with protease inhibitors (HBI): HB containing 60µg/ml chymostatin, 5 µg/ml leupeptin, 10 µg/ml pepstatin, 4mm Pefabloc, 2µg/ml aprotinin, 0.5mM ATP, and 1mm DTT. Shortly before use add to 20ml of HB 60µl of 20mg/ml chymostatin, 100µl of 1mg/ml leupeptin, 200µl of 1mg/ml pepstatin, 476µl of 167mM Pefabloc, 4µl of 10mg/ml aprotinin, 200µl of 0.1M DTT, and 50µl of 0.1M ATP.
Steps
- After the last wash in PBS (see Section III,A, step 7) remove PBS and weigh the brains.
- Cut the brains into small pieces using a razor blade (keep brains on ice).
- Add 0.75ml of HBI per gram of wet tissue (keep brain suspension on ice).
- Transfer brain suspension into a glass homogeniser on ice.
- Grind brain tissue for 20 passages of the pestle on ice.
- Centrifuge crude extract at 15,000g for 1h at 4°C.
- Recover clarified supernatant (cytosolic brain extract) and supplement it with 150mM sucrose, 50mg/ml creatine kinase, 30mM creatine phosphate, and 0.5 mM ATP.
- Aliquot and flash freeze in liquid nitrogen. Store frozen aliquots at -80°C.
C. Preparation of Bacteria
Solutions
- Brain heart infusion (BHI) broth: Prepare liquid medium according to the manufacturer's instruction, autoclave, filter, and store at 4°C. For agar plates, add Bacto-agar (15g/liter of BHI broth), autoclave, and pour 30ml in a 10-cm petri dish. Store plates at 4°C.
- Erythromycin stock solution: Dissolve 50mg of erythromycin in 10ml of pure ethanol. Store at 4°C.
Bacterial Culture
- Streak the bacteria onto BHI agar plates. Incubate at 37°C for 24h.
- Put 5ml of BHI (supplemented with 50µg/ml erythromycin) in a 15-ml sterile Falcon tube. Scrape a few colonies off the BHI plate using a sterile pipette tip or a flamed bacteriological loop. Inoculate the broth and grow bacteria overnight at 37°C with vigorous shaking.
- Transfer bacterial culture to a centrifuge tube and pellet the bacteria at 10,000g for 3 min.
- Wash bacterial pellet three times in homogenisation buffer. After the final washing step, resuspend pellet in a final volume of homogenisation buffer corresponding to the initial volume of bacterial culture.
- Alternatively, supplement the overnight culture with 20% glycerol, aliquot, and store at -80°C.
D. Listeria Motility Assay
Solutions
- 2% methycellulose in homogenisation buffer (stock solution): Heat 100ml of HB to 60°C and then add 2g of methylcellulose. Stir vigorously until the methycellulose dissolves. Cool down and store at room temperature.
- 0.5% methycellulose in homogenisation buffer (working solution): To make 10 ml, mix 7.5 ml of HB with 2.5 ml of 2% methycellulose. Store at 4°C.
- VALAP: Mix vaseline, lanoline, and paraffin in a 1:1:1 ratio (w/w/w) and homogenise at 75°C. Store at room temperature.
- G buffer: 5mM Tris-HCl, pH 7.6, 0.5mM ATP, 0.1 mM CaCl2, and 0.5 mM DTT. For 1 litre, weigh out 0.8 g Tris-HCl, 0.01 g CaCl2, and then add 5 µl of 0.1M ATP and 5µl of 0.1M DTT. Dissolve in 900ml H2O, adjust pH to 7.6 with 1N NaOH, and bring volume to 1 litre. Store at 4°C.
- Rhodamine-labelled actin: Add 6µl of G buffer to one aliquot of rhodamine-labelled actin. Mix gently and store on ice.
Steps
- Wash bacteria three times in homogenisation buffer. Resuspend pellet in 20µl homogenisation buffer.
- In a small Eppendorf tube, mix 4 µl brain extract, 4 µl of 0.5% methycellulose, 0.5 µl bacteria suspension, and 0.2µl rhodamine-labelled actin. Mix by pipetting up and down gently. Do not vortex. Incubate mixture at room temperature for 10min.
- Remove 1.7 µl of motility mixture and spot it onto a glass slide. Gently place a 22 x 22-mm glass coverslip over the drop and press down until the drop spreads to the edges. Seal the coverslip edges with VALAP.
- Observe slide with an upright or inverted microscope. Actin comet tails can be observed easily by phase contrast or epifluorescence using a Plan- Apochromat 100x/1.4NA oil immersion objective (Figs 2 and 3).
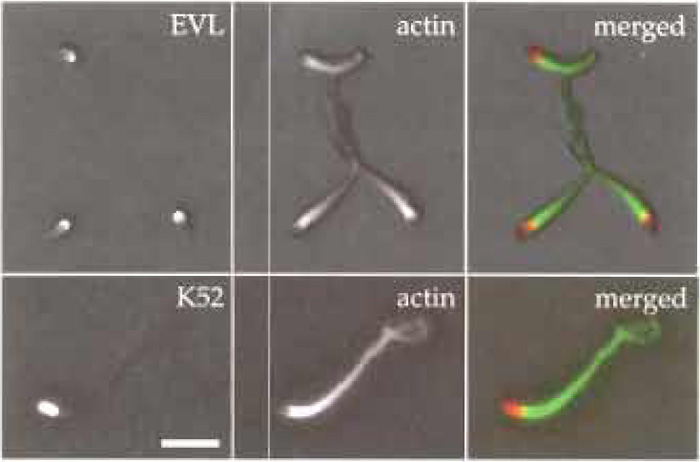 |
| FIGURE 2 Immunofluorescence microscopy showing Listeria actin tails formed in mouse brain extracts. Bacteria were incubated in mouse brain extract for 30min at room temperature and then 5µl of motility mixture was applied onto Cell Tak-coated cover slips (the coating procedure was done according to the manufacturer's instructions) and incubated for 5min on ice. Afterwards, bacteria were fixed with 4% PFA for 20min at room temperature. Immunolabelling was done according to Geese et al. (2002) using the affinity-purified polyclonal antibody K52 to label bacterial surface, the monoclonal antibody 84H1 to label EVL, and Texas redlabelled phalloidin to label the actin tails. Primary antibodies were detected using Alexa 488-conjugated secondary antibodies. Scale bar: 5 µm. |
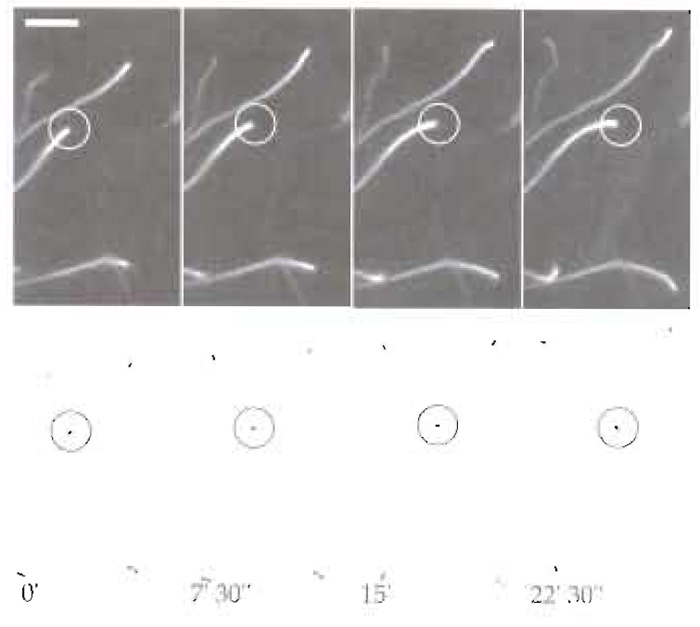 |
| FIGURE 3 Dynamics of Listeria motility in mouse brain extracts.
Bacteria were mixed with 4 µl brain extract, 4 µl of 0.5% methycellulose,
and 0.2 µl rhodamine-labelled actin and were incubated at room
temperature for 10min. Thereafter, 1.7µl of motility mixture was
spotted onto a glass slide and a 22 × 22-mm glass coverslip overlaid
on it. Bacterial motility was observed using a Axiovert 135 TV
inverted microscopy equipped with phase-contrast and epifluorescence
optics using a Plan-Apochromat 100×/1.4NA oil immersion
objective. Images were acquired using a cooled, back-illuminated
CCD camera (TE/CCD-1000 TKB; Roper Scientific) driven by IPLab
Spectrum software (Scanalitics). (Top) Rhodamine-labelled actin and
(bottom) the corresponding phase-contrast images. Scale bar: 10µm. |
IV. COMMENTS AND PITFALLS
Although cell-free systems based on egg extracts of Xenopus laevis or human platelet extracts provide excellent in vitro systems for supporting actin-based bacterial motility, their ability to do so can be affected negatively by various factors, such as health of eggs or quality and age of platelet preparations. In this context, mouse brain extracts offer a more "robust" in vitro system that does not seem to be influenced by external factors such as mouse strain and age.
The reconstitution of Listeria motility using mouse brain extracts can have a variety of uses. For instance, it can be used to study the role of actin cytoskeletal components involved in Listeria motility by interfering with their function using specific inhibitors or antibodies, as described in May et al. (1999). Moreover, the procedure described here may be further developed and adapted to obtain cell-free extracts from normal cultured cells or cells that lack or express mutated versions of the actin cytoskeletal proteins of interest, thus widening the spectrum of in vitro systems available for studying bacterial motility.
Three main parameters have to be considered to achieve optimal Listeria motility with mouse brain extracts. First, the protein ActA must be expressed at high levels on the bacterial surface. As most wild-type strains express low levels of this protein under standard culture conditions, a Listeria strain (see Lingnau et al., 1996) that constitutively expresses high levels of this protein must be used in this assay. The second critical parameter is the total protein concentration of the brain extract, which must be at least 10mg/ml. The total protein concentration can be increased by reducing the amount of homogenisation buffer per gram of wet tissue. Moreover, mouse brain extracts should not be diluted more than fourfold as further dilution leads to loss of activity in the motility assay. Finally, the high amount of actin present in these extracts induces its spontaneous polymerisation, characterised by the formation of short actin bundles. As this actin network may affect bacterial motility, mouse brain extracts must be kept on ice to reduce the tendency of actin to polymerise.
Acknowledgments
I thank David A. Monner and Jürgen Wehland (GBF, Department of Cell Biology) for helpful discussions and valuable support.
References
Cossart, E, and Bierne, H. (2001). The use of host cell machinery in the pathogenesis of Listeria monocytogenes. Curr. Opin. Immunol. 13, 96-103.
Frischknecht, F., and Way, M. (2001). Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 11, 30-38.
Geese, M., Loureiro, J. J., Bear, J. E., Wehland, J., Gertler, F. B., and Sechi, A. S. (2002). Contribution of Ena/VASP proteins to intracellular motility of Listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization. Mol. Biol. Cell 13, 2383-2396.
Laurent, V., and Carlier, M. F. (1998). Use of platelet extracts for actinbased motility of Listeria monocytogenes. In "Cell Biology: A Laboratory Handbook" (J. Celis, ed.), pp. 359-365. Academic Press, New York.
Laurent, V., Loisel, T. P., Harbeck, B., Wehman, A., Grobe, L., Jockusch, B. M., Wehland, J., Gertler, F. B., and Carlier, M. F. (1999). Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J. Ceil Biol. 144, 1245-1258.
Lingnau, A., Chakraborty, T., Niebuhr, K., Domann, E., and Wehland, J. (1996). Identification and purification of novel internalin- related proteins in Listeria monocytogenes and Listeria ivanovii. Infect. Immun. 64, 1002-1006.
Loisel, T. P., Boujemaa, R., Pantaloni, D., and Carlier, M. F. (1999). Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613-616.
Marchand, J. B., Moreau, P., Paoletti, A., Cossart, P., Carlier, M. F., and Pantaloni, D. (1995). Actin-based movement of Listeria monocytogenes: Actin assembly results from the local maintenance of uncapped filament barbed ends at the bacterium surface. J. Cell Biol. 130, 331-343.
Niebuhr, K., Ebel, F., Frank, R., Reinhard, M., Domann, E., Carl, U. D., Walter, U., Gertler, F. B., Wehland, J., and Chakraborty, T. (1997). A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 16, 5433-5444.
Pistor, S., Chakraborty, T., Walter, U., and Wehland, J. (1995). The bacterial actin nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins. Curr. Biol. 5, 517-525.
Pistor, S., Grobe, L., Sechi, A. S., Domann, E., Gerstel, B., Machesky, L.M., Chakraborty, T., and Wehland, J. (2000). Mutations of arginine residues within the 146-KKRRK-150 motif of the ActA protein of Listeria monocytogenes abolish intracellular motility by interfering with the recruitment of the Arp2/3 complex. J. Cell Sci. 113, 3277-3287.
Skoble, J., Auerbuch, V., Goley, E. D., Welch, M. D., and Portnoy, D. A. (2001). Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 155, 89-100.
Skoble, J., Portnoy, D. A., and Welch, M. D. (2000). Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell Biol. 150, 527- 538.
Small, J. V., Stradal, T., Vignal, E., and Rottner, K. (2002). The lamellipodium: Where motility begins. Trends Cell Biol. 12, 112-120.
Smith, G. A., Theriot, J. A., and Portnoy, D. A. (1996). The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J. Cell Biol. 135, 647-660.
Theriot, J. A., Rosenblatt, J., Portnoy, D. A., Goldschmidt-Clermont, P. J., and Mitchison, T. J. (1994). Involvement of profilin in the actin-based motility of L. monocytogenes in cells and in cell-free extracts. Cell 76, 505-517.




