Determination of Alkalinity of Water
To determine the amount of the following types of alkalinity present in the given samples:
- Hydroxide alkalinity
- Carbonate alkalinity
- Bicarbonate alkalinity
- Hydroxide–Carbonate alkalinity
- Carbonate–Bicarbonate alkalinity
Principle
The alkalinity of water is a measure of its capacity to neutralize acids. It is primarily due to salts of weak acids, although weak or strong bases may also contribute. Alkalinity is usually imparted by bicarbonate, carbonate and hydroxide. It is measured volumetrically by titration with 0.02 N sulphuric acid and is reported in terms of CaCO3 equivalent. For samples whose initial pH is above 8.3, the titration is conducted in two steps. In the first step, the titration is conducted until the pH is lowered to 8.2, the point at which phenolphthalein indicator turns from pink to colourless. This value corresponds to the points for conversion of carbonate to bicarbonate ion. The second phase of titration is conducted until the pH is lowered to 4.5, corresponds to methyl orange end point, which corresponds to the equivalence points for the conversion of bicarbonate ion to carbonic acid.
1. Burette
2. Erlenmeyer flask
3. Pipettes
Reagents (click to check the preparation of reagents)
1. Carbon dioxide free distilled water.
2. Phenolphthalein indicator.
3. Methyl orange indicator.
4. 0.1 N sodium thiosulphate solution
5. 0.02 N sulphuric acid.
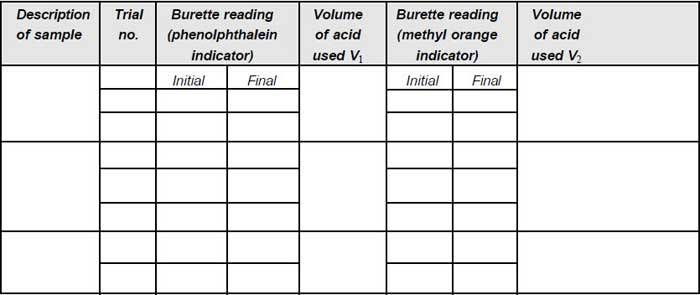
1. Pipette 50 mL of sample into a clean Erlenmeyer flask (V).
2. Add one drop of sodium thiosulphate solution, if residual chlorine is present.
3. Add two drops of phenolphthalein indicator; if the pH is above 8.3, colour of solution becomes pink.
4. Titrate against standard sulphuric acid in the burette, till the colour just disappears. Note down the volume (V1).
5. Then add two drops of methyl orange indicator, the colour turns yellow.
6. Again titrate against acid, until the colour turns to orange yellow. Note down the total volume (V2).
Observation
0.02 N H2SO4 x sample (Methyl orange/phenolphthalein indicator)
Calculation
| 1. Phenolphthalein alkalinity (P) as mg/L CaCO3 = | V1 x 1000 |
| mL of sample |
| 2. Total alkalinity (T) as mg/L CaCO3 = | V2 x 1000 |
| mL of sample |
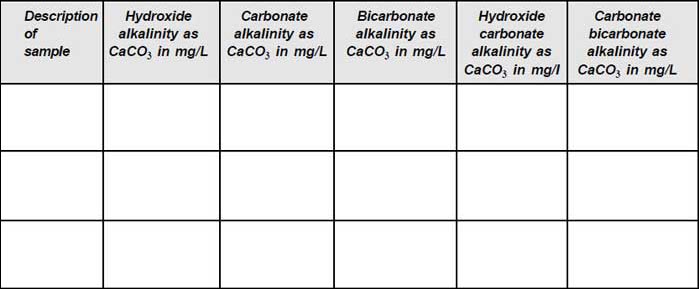
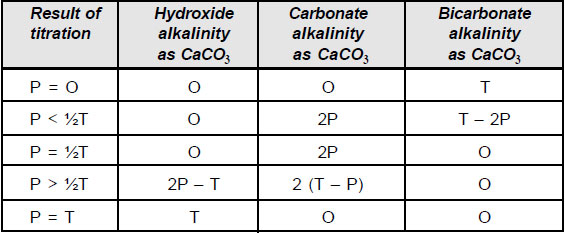
The types of alkalinities present in the samples are calculated using the equations given in the following table and the results are tabulated.
Results