Determination of Kjeldahl Nitrogen
To determine the Kjeldahl nitrogen of the given sample of water.
Principle
In the presence of sulphuric acid, potassium sulphate and mercuric sulphate catalyst, the amino nitrogen of many organic materials is converted to ammonium sulphate. After the mercury-ammonium complex, the digestible has been decomposed by sodium thiosulphate, the ammonia is distilled from an alkaline medium and absorbed in boric acid. The ammonia is determined colorimetrically or by titration with a standard mineral acid.
- Digestion apparatus of 800 mL capacity
- Distillation apparatus
- Spectrophotometer
Reagent (click to check the preparation of reagents)
- All reagents listed for the determination of ammonia N
- Digestion reagent
- Phenolphthalein indicator
- Sodium hydroxide-sodium thiosulphate reagent
- Borate buffer solution
- Sodium hydroxide 6N
Procedure
- Place a measured sample into a digestion flask. Dilute the sample to 300 mL, and neutralise to pH 7.
Sample size is determined as follows: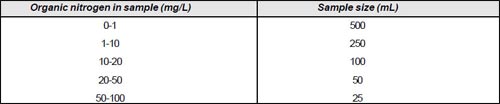
- Add 25 mL borate buffer and 6 N NaOH until pH 9.5 is reached.
- Add a few glass beads and boil off 300 mL.
- Cool and add carefully 50 mL digestion reagent. After mixing heat under a hood until the solution clean to a pale straw colour.
- Digest for another 30 minute and allow the flask and contents cool.
- Dilute the contents to 300 mL and add 0.5 mL phenolphthalein solution.
- Add sufficient hydroxide-thiosulphate reagent to form an alkaline layer at the bottom of the flask.
- Connect the flashed to the steamed out distillation apparatus and more hydroxide-thiosulphate reagent.
If a red phenolphthalein colour fails to appear at this stage. - Distilled and collect 200 mL distillate below the surface of boric acid solution. Extend the lip of condenser well bellow the level of boric acid solution.
- Determine the ammonia as described earlier by taking 50 mL portion of the distillate.
- Carry out a similar procedure for a blank and apply the necessary correction.
The observation is presented in Tables A and B respectively.
Table A: Observation for calibration

Table B:

| Organic N in mg/L = | A x 1000 | x B and C |
| mL of sample |
where,
A = mg N found colorimetrically
B = mL of total distillate collected including H3BO3
C = mL of distillation taken for Nesslerisation.
Results