Cytochrome P450
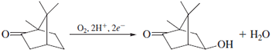 |
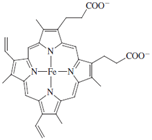
At the core of cytochrome P450 is an iron porphyrin, or heme, group, protoporphyrin IX, which is depicted below.
In the structure in Fig. 9, the iron porphyrin is shown in black.
Cytochrome P450 is a redox catalyst. The multiple available oxidation states allow the cofactor to accept and donate electrons during different stages of the catalytic cycle. Since the early 1970s, this enzyme and its relatives have been the subject of intense study by, among others, enzymologists, toxicologists, biophysical chemists, and inorganic chemists. The latter have tried to model the chemistry of cytochrome P450 with synthetic small molecules with the twin goals of mimicking its activity and understanding how the enzyme itself works. Working in parallel, biophysical chemists and enzymologists have performed many steady-state and pretransient kinetic studies such as the ones already discussed, which have contributed to a working model for the mechanism shown in Fig. 10.
 |
One important line of investigation which has supported the radical rebound hypothesis is the use of radical clock substrate probes. These probes rearrange in a diagnostic way on a very rapid and calibrated time scale when a hydrocarbon radical is formed. In the case of P450, rearranged products have been isolated after oxidation and have been used as evidence of an intermediate substrate radical. In this way, even though the lifetime of the radical is too short for it to be observed directly, its character can be explored by the judicious choice of substrate analogues.
Mechanistic proposals are under constant scrutiny and revision, and aspects of the foregoing mechanism have been challenged. In particular, the possibility has been suggested that a species other than a high valent iron-oxo (likely a hydroperoxo species) may be the active oxidant for some substrates. Debates such as these are a great strength of the study of enzyme mechanisms. Given all the tools which have been developed in this field, and the wealth of interesting problems to which these tools can be applied, the study of enzyme mechanisms should be considered a vital and evolving process. The answer to the question of “how enzymes work” cannot be described fully in a single scheme.
![The cytochrome P450cam structure. The bound heme is depicted in black, and the iron atom at the center of the heme appears as a sphere. [Adapted from Poulos, T. L., Finzel, B. C., and Howard, A. J. (1986). “Crystal structure of substrate-free Pseudomonas putida cytochrome P450,” Biochemistry 25, 5314–5322.]](images/2-9.gif) |
| Figure 9 The cytochrome P450cam structure. The bound heme is depicted in black, and the iron atom at the center
of the heme appears as a sphere. [Adapted from Poulos, T. L., Finzel, B. C., and Howard, A. J. (1986). “Crystal structure of substrate-free Pseudomonas putida cytochrome P450,” Biochemistry 25, 5314–5322.] |




