Derivatives—Natural and Laboratory
|
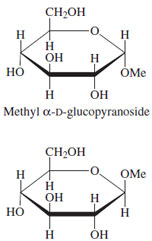
| Figure 11 Methyl glucopyranosides. |
The hydroxyl group formed as a result of ring closure
represents a site for attachment of a broad variety of
substituents. Compounds that are formed in such reactions
are full acetals (ketals) and thus no longer undergo
interconversion at the anomeric center. The configuration
of such glycosides is therefore either alpha or beta depending
on the relationship between the C-1 group and
the projection of the ring; if on the same side, designate
alpha, otherwise, beta (Fig. 11). A wide variety of natural
and man-made derivatives (glycosides) are known with
the substituents, aglycones, varying from simple methyl
groups to complex organic molecules including other sugars
(see below).
In addition to substitution of the anomeric hydroxyl,
many modifications of the hydroxyl loci are known in
nature. Most prevalent are those in which the hydroxyl
group at C-2 is replaced by an amino function, generally
acetylated. The sugar 2-deoxy2-acetamido-D-glucose (
N-acetylglucosamine)
is distributed throughout nature and,
in its polymeric form (chitin), forms the organic matrix of
insect and arthropod exoskeletons. Hence, it is likely the
second most prevalent organic molecule on earth. Other
variations include oxidation (C-6 or C-1) to form carboxyl
groups and loss of a hydroxyl to form deoxy sugars
(Fig. 12).
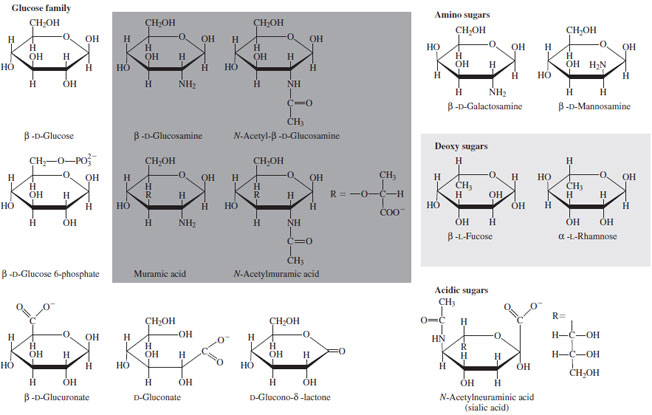 |
| Figure 12 Sugars related to D-glucose that occur in nature. |