Basic questions about kinetochore function

Fig. 8.10. The process of microtubule attachment to the kinetochore. (a) Several microtubules grow out from, and shrink back towards, the spindle pole during prometaphase. (b) One microtubule makes a lateral contact with a kinetochore. The kinetochore attaches to it and starts moving polewards. (c) A vesicle makes a lateral contact with the same microtubule and also moves polewards.
(i) how do microtubules get attached to kinetochores? and
(ii) w.hat are the structural requirements for the generation of the force required for chromosome movement? Both these questions have been partly answered through experiments conducted in recent years (1990 and 1991).
Mechanism of kinetochore-microtubule attachment.
Following two hypotheses were earlier available for kinetochore-microtubule attachment : (i) According to first hypothesis, the microtubules are nucleated at the centrosome and later captured by the kinetochores. (ii) According to second hypothesis the microtubules are nucleated at the kinetochores, and only later interact with the spindle pole. Recently, support for the first hypothesis was provided by video contrast optical microscopy. It was possible to image individual microtubules, as they grow out from mitotic poles and first come in contact with kinetochores.

Fig. 8.10. The process of microtubule attachment to the kinetochore. (a) Several microtubules grow out from, and shrink back towards, the spindle pole during prometaphase. (b) One microtubule makes a lateral contact with a kinetochore. The kinetochore attaches to it and starts moving polewards. (c) A vesicle makes a lateral contact with the same microtubule and also moves polewards.
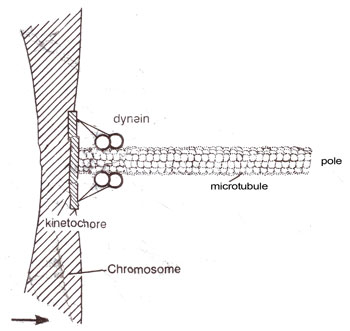
Fig. 8.11. Possible arrangement of dynein in the kinetochore. Two headed dynein molecules are shown tugging on the end of a microtubule, whose disassembly could govern rate of movement.
According to an earlier view, the kinetochore is a passive structure, which remains attached tomicrotubule ends, while these mfcrotubules are shortened. The shortening of microtubules is achieved by disassembly or depolymerization. In this hypothesis, energy released (from GTP hydrolysis) during the microtubule assembly and disassembly is sufficient to drive chromosome movement during anaphase A, and any role of motor molecules was ruled out. However,

Fig. 8.11. Possible arrangement of dynein in the kinetochore. Two headed dynein molecules are shown tugging on the end of a microtubule, whose disassembly could govern rate of movement.
In vitro association and relative movement of microtubules and kinetochores Recently the forces involved in chromosome movements have been studied using in vitro techniques involving association of isolated chromosomes and microtubules.
It was shown that microtubules become associated with kinetochores and in presence of ATP (to add phosphate group) travel towards the minus end (of in poleward direction). The rate of movement was 0.46 μm sec-1 (28 μmmin-1), similar to that in early prometaphase, but much greater than the known rate of chromosome movement during anaphase. This was explained on the basis of the assumption that chromosomes were perhaps isolated from cells arrested at prometaphase. The movement can be attributed to cytoplasmic dynein.
Association after pretreatment with ATP-v-S.
When the chromosomes and microtubules were pretreated with ATP-ν-S (with thiophosphate group) before adding ATP, the movement was much slower (.048 μm/sec-1) and in opposite direction i.e. towards the centre.
Using in virto studies, it could be shown that there are two typesof motor proteins at the kinetochores, one developing minus-end directed force and the other developing a plus-end directed force. ATP analogues (e.g. ATP-ν-S) were used which are known to be used differently by known motor proteins, dynein and kinesin, both ATPases. The minus-end motor gives a response similar to that given by dynein, which has now been shown to be located at kinetochore. But plus-end motor protein seems to be different from kinesin (GTP is used by kinesin, but not by plus-end motor protein; also kinesin makes microtubules move much faster), which unlike dynein could not be located in kinetochore. Thus it may be a new protein, likely to be identified in the near future. It has also been shown that differential phosphorylation and dephosphorylation of two ATPases (motor protons) in kinetochores are responsible for chromosome movements during cell division.




