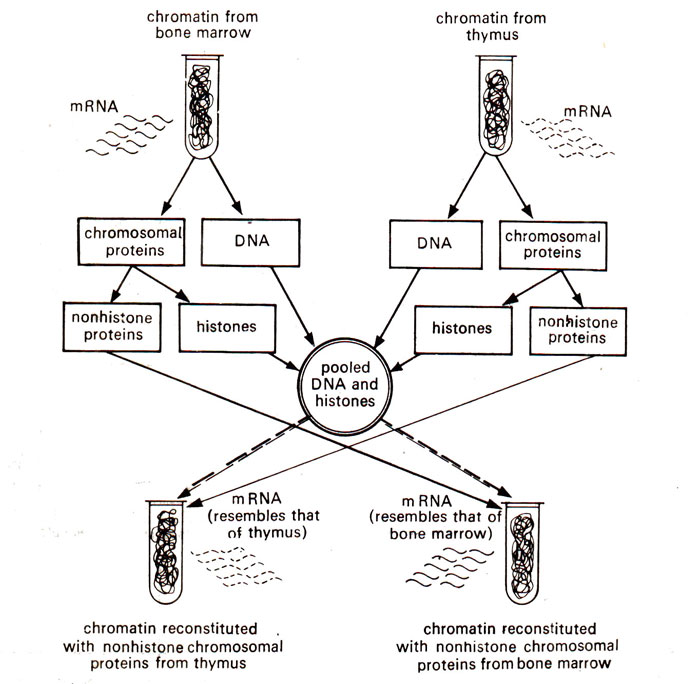
Fig. 37.4. Chromatin reconstitution experiment demonstrating the positive role of non-histone proteins.
As we know, chromosomal proteins are mainly of two kinds : (i)
histones and
(ii) non-histones, so that the chromatin mainly has three components- DNA, histones and non-histones. While it is known since early 1960's that histones may be involved in repressing gene activity, the specific regulation by non-histones was demonstrated only during 1970s. The most important experiments in this connection were pioneered by
Gilmour and
Paul (1973). They isolated the chromatin from different tissues separately and then dissociated it into DNA, histones and non-histones.
This was followed by chromatin reconstitution using either the three components derived from chromatin of the same tissue or by combining non-histones of one tissue with the DNA and histones of another tissue (Fig. 37.4). It could be demonstrated in such experiments that the mRNA which is synthesized
in vitro from reconstituted chromatin, mainly depended on the source of non-histone proteins (for a review consult O'Malley
et al., 1977).

Fig. 37.4. Chromatin reconstitution experiment demonstrating the positive role of non-histone proteins.
There are atleast three specific proteins, whose synthesis in eukaryotes has been studied using chromatin reconstitution experiments. These are (i) the globin synthesized in reticulocytes, (ii) the ovalbumin synthesized in chick oviduct due to hormonal stimulation and (iii) the histones synthesized during 'S' phase of cell cycle. In all these three cases, similar observations were available which are as follows : (i) Same quantity and quality of mRNA was synthesized on native chromatin as well as on chromatin reconstituted from DNA, histones and non-histones derived from same tissue, (ii) When heterologous chromatin was reconstituted by using non-histones from one tissue and DNA and histones from another tissue, mRNA synthesized was similar to that synthesized on the native chromatin to which non-histones belonged (Fig. 37.4).
Above observations suggested a positive regulatory role of non-histones in eukaryotes. This role of non-histones is also suggested by the following additional information, (i) Non-histones are present in increased quantity in tissues active in RNA synthesis, while quantity of histones is constant, (ii) Non-histones exhibit 2-3 times more diversity than histones, (iii) Non-histones have tissue specificity and DNA binding specificity. (iv) Certain non-histones are known to induce RNA synthesis
in vitro, (v) Synthesis of specific non-histones is associated with induction of gene activity, (vi) A large number of transcription factors earlier described in
Expression of Gene : Protein Synthesis 2. Transcription in Prokaryotes and Eukaryotes and to be later described in this section, are actually non-histone proteins.
Above observations though suggest a specific role of non-histones, but in the absence of histones, non-histones have been shown not to induce RNA synthesis. This suggests that regulation of protein synthesis actually involves an interaction between histones and non-histones, where histones inhibit protein synthesis non-specifically and non-histones induce RNA synthesis rather specifically. Some histone proteins may also function as transcription factors.
Transcription complex and activation of smart genes. It has now been established through a variety of studies, that for the elaborate transcription process to get underway, several proteins (called transcription factors) must bind to the DNA sequences near a target gene. This forms a transcription complex, which works as 'brain' of a smart gene and is comparable to a 'computer'. In this brain or computer, the signals are combined to make a decision about whether or not to switch on the smart gene. Only by the right combination of transcription factors or signals, the transcriptional enzyme (RNA polymerase) is activated, and produces RNA. In contrast to this computer like complex control of transcription in eukaryotes, the control in prokaryotes is simple and can be compared to mere switches.
In eukaryotes, a number of DNA binding proteins and their corresponding binding regions on DNA have been identified and isolated in a variety of organisms. While in prokaryotes, there is a maximum of three protein binding sites near the transcription start point, in eukaryotes, these sites may be atleast five and may be as many as 20 for some smart genes (not for house keeping genes). For instance, in sea urchin, for the regulation of a gene producing actin, as many as 20 DNA regions near this gene have been identified, which are recognized by regulatory proteins. Atleast five of these 20 sites are used for activation of actin gene.
In
Expression of Gene : Protein Synthesis 2. Transcription in Prokaryotes and Eukaryotes, we described seven transcription factors
(TFIID, TFIIA, TFIIB, TFIIE, TFIIF, TFIIJ, TFIIH), which are essential for transcription of all genes including
house keeping genes and
smart genes. However, for transcription of smart genes, additional transcription factors must form a complex to activate the obligatory proteins coupled to the RNA polymerase II enzyme that transcribes the gene into hnRNA. Only when all components (including the obligatory proteins and the right combination of transcription factors forming a complex) are present, the transcription of a smart gene will take place. Several hundred different transcription factors have been found to date, two third of these falling into four recognizable groups—
helix-turn-helix, helix-loop-helix, zinc finger and
leucine zipper. The remaining one third may be unique to their types. As discussed in
Expression of Gene : Protein Synthesis 2. Transcription in Prokaryotes and Eukaryotes, each transcription factor has separate domains for (i) recognition and binding a specific DNA sequence and for (ii) activation of obligatory proteins. These are called
DNA binding domain and
activation domain.
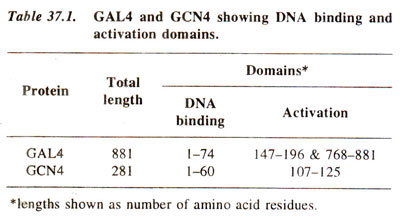
Some of the earliest studied transcription factors include GAL4 and GCN4 in yeast, which activate genes involved in metabolism of galactose and those involved in general control of nitrogen metabolism (amino acid biosynthesis) respectively. The details of these two transcription factors are shown in Table 37.1.
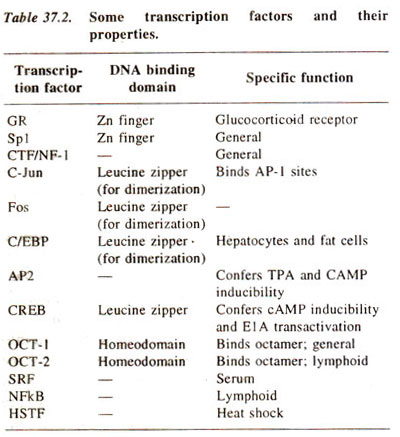
A number of other transcription factors are listed in Table 37.2. The mechanism involved in binding of these transcription factors to DNA and their involvement in protein-protein interactions was discussed in
Expression of Gene : Protein Synthesis 2. Transcription in Prokaryotes and Eukaryotes. It should be recognized that the discovery of regulatory DNA sequences and transcription factors was done independent of each other in many cases, so that in all cases it is not known, which transcription factor regulates the activity of which specific genes. However, certain transcription factors are known to be found in some specialized cells and not in others, so that some idea about the genes regulated by them can be inferred. In other cases, where genes have been isolated, their regulatory sequences could also be identified. Similarities are available among the different regulatory DNA sequences as well as among amino acid sequences of different transcription factors, suggesting mechanisms of DNA-protein interactions (see
Expression of Gene : Protein Synthesis 2. Transcription in Prokaryotes and Eukaryotes).