Lipids
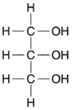 |
| Figure 4-21 Structural formula for glycerol. |
Fats are complex molecules composed of long chains of carbon atoms (fatty acids) linked to a 3-carbon compound called glycerol. The structure of glycerol is quite simple, as shown in figure 4-21. As previously shown, organic acids each have a carboxyl group (−COOH) at the end of a chain of carbon atoms. A fatty acid is shown in figure 4-22.
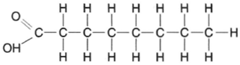 |
| Figure 4-22 Structural formula for a fatly acid. |
When fatty acids react with glycerol to produce molecules of fat, a dehydrolysis reaction takes place. Three molecules of water are removed (or created) for each molecule of fat manufactured, as shown in figure 4-23. As illustrated in the figure, the carbon atoms are linked to each other by single bonds, the remaining bonds being attached to hydrogen atoms. This is called a saturated fat. An unsaturated fat has fewer hydrogen atoms and, hence, some double bonds between adjacent carbon atoms, as shown in figure 4-24. An unsaturated fat can be changed to a saturated fat by hydrogenation, the process of adding hydrogen atoms to a compound.
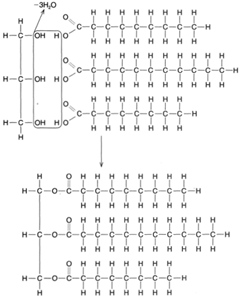 |
| Figure 4-23 A dehydrolysis reaction uniting glycerol and three fatty acid molecules to form a molecule of fat. |
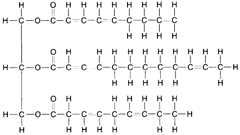 |
| Figure 4-24 Structural formula for an unsaturated fat. |




