Organic Acids
If the terminal carbon atom of such a series bonds with an oxygen atom and an −OH group, as shown in figure 4-8, an organic acid is created. The group −COOH is called a carboxyl group (figure 4-9). Note that in all cases the carbon atom always has four bonds, the hydrogen atom always one bond, and the oxygen atom always two bonds.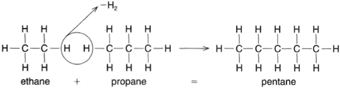 |
| Figure 4-10 A dehydrogenation reaction causing the linkage of ethane and propane to produce pentane. |
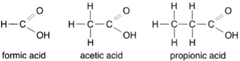 |
| Figure 4-9 Structural formulas for formic acid, acetic acid, and propionic acid. |
Two organic compounds of this type can react together to form a longer chain, as shown in figure 4-10. Because hydrogen is removed to create these compounds, this is referred to as a dehydrogenation reaction.




