Infections in immunocompromised patients
Medical treatment or hereditary deficiency of components of the immune system may allow organisms with reduced virulence to cause infection and normal pathogens to cause severe infection. The origin of immune deficiency is often multifactorial; for example patients undergoing bone marrow transplantation are neutropenic, which reduces resistance to bacterial infection, whilst intravenous cannulation provides a route for Staphylococcus epidermidis infection.Neutropenia
Neutropenia most often arises as a result of acute leukaemia or its treatment; infection risk depends on the duration and severity of the neutropenia. Bacteraemia occurs in between 40 and 70% of neutropenic patients with Enterobacteriaceae and Pseudomonas spp. invading following gut damage by antineoplastic agents or irradiation. Gram-positive organisms (e.g. S. epidermidis, Streptococcus mitis, Streptococcus oralis, Enterococcus spp., Staphylococcus aureus and Corynebacterium jeikeium) are increasingly important causes of sepsis. With increasing duration of neutropenia, fungal infection may develop due to Candia albicans, Aspergillus spp. and, rarely, Fusarium spp., Pseudallescheria boydii and Trichosporon beigelii.
Empirical therapy includes tazobactam and an aminoglycoside, with vancomycin being added if central-line infection is possible. If fever persists, fungal pathogens are more likely and amphotericin or itraconazole may be added.
Prevention of infection
Infection risk can be reduced by:
- source isolation (see Principles of infection control);
- filtration of room air to remove fungal spores;
- antifungal prophylaxis (e.g. nystatin, either alone or in combination with oral amphotericin, or fluconazole or itraconazole);
- antibiotic prophylaxis (e.g. fluoroquinolones are used in some centres).
T-cell deficiency
T-cell deficiency is an increasingly common problem, which may follow HIV infection, cancer chemotherapy, corticosteroid therapy or organ transplantation. Congenital T-cell deficiencies are rare but may be purely linked to T-cell function or combined with hypogammaglobulinaemia.
These are mainly the microorganisms that have an intracellular location in the human host, such as:
- Toxoplasma gondii, Strongyloides stercoralis;
- Mycobacterium tuberculosis, Mycobacterium avium- intracellulare;
- Listeria monocytogenes, Cryptococcus neoformans, Pneumocystis jiroveci;
- herpes simplex, cytomegalovirus (CMV), varicella zoster virus (VZV) and measles.
Measles infection, especially if complicated by giant cell pneumonia or encephalitis, can be life-threatening.
Diagnosis
Specific infections should be investigated appropriately. All patients should have at least two blood cultures taken from different sites.
Hypogammaglobulinaemia
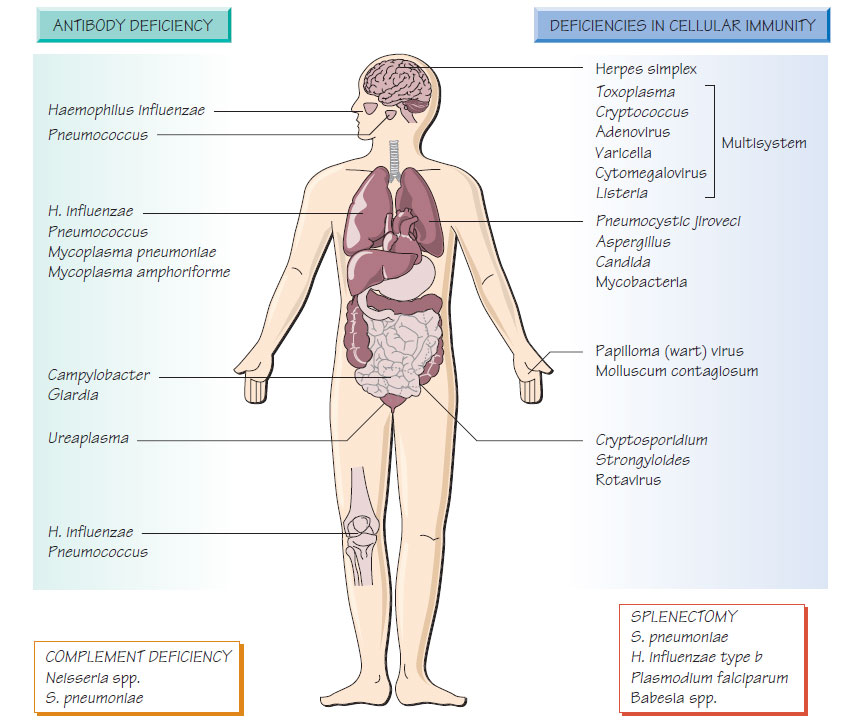
Patients with X-linked agammaglobulinaemia are at increased risk of infection for the first 6 months of life; patients with common variable immunodeficiency are at increased risk throughout life. Functional hypogammaglobulinaemia develops in patients with multiple myeloma. Patients suffer recurrent respiratory tract infections with Streptococcus pneumoniae, the novel Mycoplasma amphoriforme and non-capsulate Haemophilus influenzae, which lead to bronchiectasis. Giardia, Cryptosporidium and Campylobacter infections may be more persistent. Intravenous immunoglobulin reduces recurrent infection.
Hereditary complement deficiencies are rare. Deficiency in the later components of the complement cascade (C7-9) results in an inability to lyse Gram-negative bacteria, and patients are susceptible to recurrent Neisseria infection. Deficiency of the alternative complement pathway leads to serious S. pneumoniae infections, including meningitis. Acquired complement deficiency occurs in systemic lupus erythematosus.
Mannose-binding lectin
A wide range of bacteria fungi, viruses and protozoa bind to mannose-binding lectin and there are reports that infection with these organisms are commoner or more severe with some deficiency genotypes.
Postsplenectomy infection
The incidence of serious sepsis following splenectomy is about 1% per year; the rate is higher in infants and children. The highest mortality is associated with splenectomy for lymphoma and thalassaemia. Patients with sickle cell disease have functional asplenia. Although the risk of sepsis diminishes with time, it never disappears. Streptococcus pneumoniae is responsible for approximately twothirds of infections; others H. influenzae and Escherichia coli. Malaria may run a fulminant course. Splenectomy predisposes to Capnocytophaga canimorsus infection, which usually arises after a dog bite.
- Conjugate vaccines against S. pneumoniae meningococcus and H. influenzae type b.
- Low-dose, oral antibiotic prophylaxis with penicillin V.
- Patient awareness of the urgency of treating respiratory infections with antibiotics, which may be prescribed in advance to avoid delay in initiation of treatment.
Pneumocystis jiroveci
Pneumocystis jiroveci is a fungus causing infection in individuals with severe T-cell dysfunction. Transmitted by the respiratory route, P. jiroveci adheres strongly to pneumocytes.
Clinical presentation
- Dyspnoea develops insidiously over days or weeks.
- Patients have an unproductive, dry cough.
- Pleuritic chest pain is uncommon.
- Patients are febrile but chest examination usually normal, although fine basal crackles may be heard.
- Chest X-ray may appear normal initially then develop through reticular shadowing until finally there is diffuse air-space consolidation.
Specimens, obtained by bronchoalveolar lavage or sputum induction may be examined by specific immunofluorescence, or nucleic acid amplification test (NAAT).
Treatment
Treatment is with oral co-trimoxazole in high dosage or intravenous pentamidine. Alternatives include trimethoprim-dapsone, pyrimethamine-clindamycin and atovaquone.
Toxoplasma infection persists inside the host cells for very long periods. Falling immunity allows the reactivation of previously dormant infection. A space-occupying lesion may develop in the brain, which may be accompanied by encephalitis.
Toxoplasma encephalitis presents with fever and headaches. Convulsions, coma and focal neurological signs may follow. A CT scan may demonstrate multiple diagnostic focal lesions with ring enhancement. Brain biopsy may yield material for tissue culture or PCR. Toxoplasma encephalitis is treated with pyrimethamine- sulfadiazine. Long-term suppressive treatment is required after recovery.