Simple β-Carboline Alkaloids
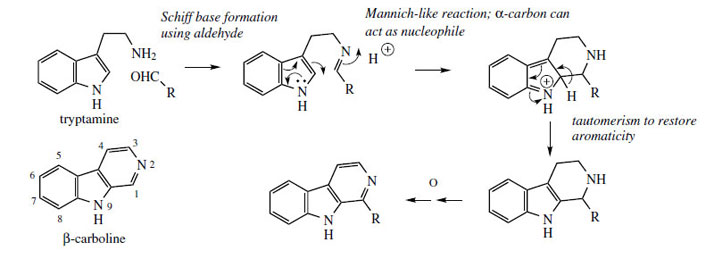
Alkaloids based on a β-carboline system (Figure 73) exemplify the formation of a new sixmembered
heterocyclic ring using the ethylamine
side-chain of tryptamine in a process analogous to
generation of tetrahydroisoquinoline alkaloids. Position 2 of the indole system is nucleophilic
due to the adjacent nitrogen, and can participate
in a Mannich/Pictet–Spengler type reaction, attacking a Schiff base generated from tryptamine
and an aldehyde (or keto acid) (Figure 73). Aromaticity
is restored by subsequent loss of the
C-2 proton. (It should be noted that the analogous
chemical reaction actually involves nucleophilic
attack from C-3, and then a subsequent
rearrangement occurs to give bonding at C-2;
there is no evidence yet for this type of process
in biosynthetic pathways.) Extra carbons
are supplied by aldehyes or keto acids, according
to the complexity of the substrate (compare
tetrahydroisoquinoline alkaloids, page 321).
 |
| Figure 73 |
 |
| Figure 74 |
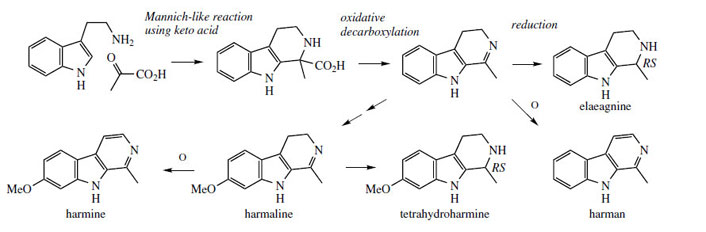
Thus,
complex β-carbolines, e.g. the terpenoid indole
alkaloid ajmalicine, are produced
by a pathway using an aldehyde such as secologanin.
Simpler structures employ keto acids,
e.g.
harmine (Figure 74) incorporates two extra
carbons from pyruvate. In such a case, an acid
is an intermediate, and oxidative decarboxylation
gives the dihydro-β-carboline, from which reduced tetrahydro-β-carboline structures, e.g.
elaeagninefrom
Elaeagnus angustifolia (Elaeagnaceae), or
fully aromatic β-carboline structures, e.g.
harman and
harmine from
Peganum harmala (Zygophyllaceae)
are derived (Figure 74). The methoxy
substitution in the indole system of harmine is
introduced at some stage in the pathway by successive
hydroxylation and methylation reactions.
A sequence from 6-hydroxytryptamine is also feasible.
The reported psychoactive properties of the
plants
Peganum harmala and
Banisteriopsis caapi(Malpighiaceae) is due to the β-carboline alkaloids
such as harmine, harmaline, and tetrahydroharmine
(Figure 74).






