 |
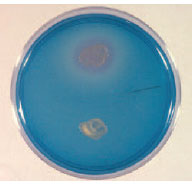
| Plate 21 DNase test. When a plate containing DNA is flooded with toluidine blue, the colony of deoxyribonuclease-producing organisms (top) and the surrounding area of hydrolyzed DNA become pink. |
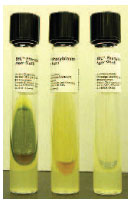 |
| Plate 22 Phenylalanine deaminase (PDase) test. The PDase-producing Providencia stuartii (left) hydrolyzes phenylalanine in the culture medium. After ferric chloride is added to the slant, the green positive reaction appears. In the middle is the PDasenegative Escherichia coli; the tube on the right is uninoculated. |
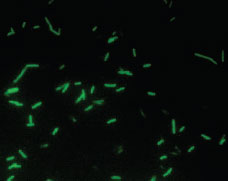 |
| Plate 23 Fluorescent antibody preparation of Legionella pneumophila viewed microscopically with an ultraviolet light source. After the patient specimen is treated with an antibody conjugated with a fluorescent dye, the brightly fluorescing bacilli are easily visible against the dark background. |
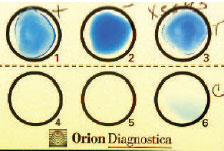 |
| Plate 24 Latex agglutination reaction. Antibody-coated latex particles have been mixed with the positive (well 1) and negative (well 2) controls and the organism isolated from the patient (well 3). The dark blue rims of the positive control and patient mixtures represent the positive reaction of agglutinated latex particles. Well 6 is an additional control. |
 |
Plate 25 A direct enzyme immunoassay for Clostridium difficile toxin. The wells have been coated with antibody against the toxin and suspensions of patient fecal specimens added. The first well in row A and the second wells in rows G and H are strongly positive, whereas the second well in row E shows a weakly positive
reaction. In the third column, positive (row A) and negative (row B) controls are shown. Refer to figure 19.3 for details of the test. |
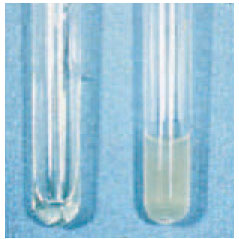 |
| Plate 26 Coagulase test. The tube of plasma on the right was inoculated with Staphylococcus aureus. A solid clot has formed in this tube in comparison to the still liquid plasma in the uninoculated tube on the left. |
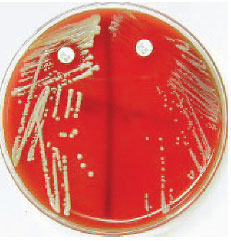 |
| Plate 27 Novobiocin disk test for differentiating two coagulase-negative species of staphylococci: Staphylococcus saprophyticus (left) and Staphylococcus epidermidis (right). The zone of inhibition around S. saprophyticus is less than 16 mm, which identifies this species by its resistance to the antibiotic. |
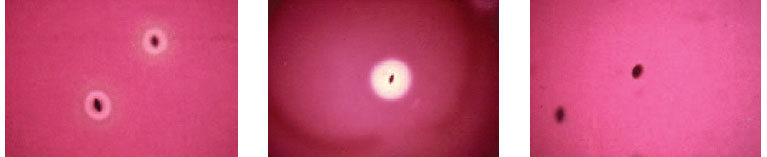 |
| Plate 28 Subsurface colonies of alpha- (left), beta- (center), and nonhemolytic (right) streptococci. Note many intact red cells and a greenish color around the alpha-hemolytic colonies. Hemolysins produced by beta-hemolytic colonies have completely destroyed surrounding red cells. Nonhemolytic organisms produce no change in the red cells. |
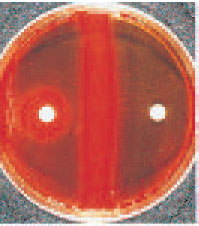 |
| Plate 29 Bacitracin test. The organism on the left is identified presumptively as Streptococcus pyogenes (group A), because it shows a zone of growth inhibition around the bacitracin disk. The bacitracin-resistant organism on the right is a betahemolytic streptococcus other than group A. |
 |
| Plate 30 CAMP test. When a group B streptococcus (Streptococcus agalactiae) is streaked at right angles to a hemolytic Staphylococcus aureus (long straight streak down middle of plate), areas of synergistic hemolysis in the shape of a beta-hemolytic arrow are formed. |
|