Examination and Qualitative Culture of Voided Urine
| Purpose | To learn simple urine culture technique and to appreciate the value of aseptic urine collection |
| Materials | Sterile urine collection vessels Liquid soap Sterile gauze sponges Sterile empty test tubes Sterile 5.0-ml pipettes Pipette bulb or other aspiration device Litmus or pH papers Blood agar plates EMB or MacConkey plates Two samples of your own urine |
Procedures
- Without special preparation of the urogenital surfaces, collect a specimen of your urine in a sterile container. Wipe the outside of the container with disinfectant and close it tightly.
- Aseptically collect a second sample of your urine, following appropriate “clean-catch” techniques described in this exercise.
- If possible, these specimens should be collected within one hour of the start of the laboratory session. If they are collected earlier, refrigerate them.
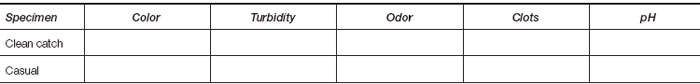
- Place about 1.0 ml of each urine sample in a small sterile test tube. Hold the tubes to the light and examine urine for color and turbidity. Test the pH of each sample with litmus or pH paper. Note the odor of each. Record your observations under Results.
- Going back to the original urine container (the test tube sample is now contaminated by the pH test), pipette a large drop of the “clean-catch” specimen onto a blood agar plate near the edge, and another drop onto an EMB or MacConkey plate. Spread the drop a little with your loop and then streak for isolation.
- Repeat step 5 with the casual urine collection.
- Incubate all plates at 35°C for 24 hours.
- Examine the incubated plates for amount of growth, types of colonies, and microscopic morphology of colony types.
Record observations under Results.
Results
- Macroscopic appearance of urine.
- Culture results.
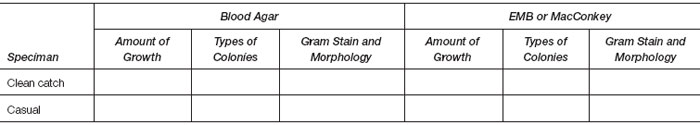
- Interpret any differences you observed in the amount of growth recovered from the two specimens.
- Interpret differences in the amount of growth on blood agar and MacConkey plates for each specimen.
- Interpret differences in the nature of growth obtained on blood agar and MacConkey plates for each specimen.
- Interpret any finding of “no growth.”




