Nitrogen Fertilizers
Soils have little capacity to retain oxidized forms of nitrogen, and ammonium accumulation in soils is small; consequently, most of the soil nitrogen is associated with organic matter. Release of nitrogen from organic matter is slow and unpredictable. If soil organic matter is depleted, as occurs in cultivated soils, nitrogen for plant growth is limited. Nitrogen is usually the most deficient nutrient in cultivated soils of the world, and fertilization of these soils with nitrogen is required. To maintain or increase productivity of soils, worldwide consumption of nitrogen fertilizers continues to increase with time (Figure 2.3). However, the consumption of phosphorus and potassium fertilizers has leveled. Anhydrous ammonia (NH3 gas) is the starting product for manufacture of most nitrogen fertilizers. Anhydrous ammonia is manufactured from hydrogen and nitrogen gases by the Haber process (Haber-Bosch process). The reaction is performed at high temperature (400 to 500°C) and high pressure (300 to 1000 atm) in the presence of a catalyst (iron or other metal) (151-153).The nitrogen gas is obtained from the air, which is about 79% nitrogen by volume, and the hydrogen is obtained from natural gas (methane), oil, coal, water, or other sources.
Jones (152) and Moldovan et al. (154) describe the production of other nitrogen fertilizers from ammonia. A brief summary of these processes follows. Nitric acid, produced from ammonia, is another basic material in the manufacture of nitrogen fertilizers. To produce nitric acid, compressed ammonia and air are heated in the presence of a catalyst and steam. The nitric acid can be reacted with ammonia to produce ammonium nitrate. Sodium nitrate is the product of the reaction of nitric acid with sodium bicarbonate. Sodium nitrate also is produced from caliche (Chilean saltpeter), which is a mineral that contains sodium nitrate and various salts of sodium, calcium, potassium, and magnesium. Sodium nitrate, sometimes called Chilean nitrate, is one of the earliest commercial nitrogen fertilizers marketed. Until 1929, all of the sodium nitrate marketed was extracted from Chilean saltpeter (154). Urea is manufactured chiefly by combining ammonia with carbon dioxide under high pressure. Ammonium sulfate is manufactured by the reaction of ammonia with sulfuric acid, gypsum, or sulfur dioxide.
The merits of nitrate and ammonium fertilizers have been researched and reviewed extensively (155–166). Many manufactured fertilizers and most organic fertilizers are ammonical; however, the ammonium that is inherent in the fertilizer or that is released upon contact with soils is soon oxidized to nitrate, unless nitrification is inhibited (167–171). Nitrification inhibitors may be employed with ammoniacal fertilizers to restrict losses of nitrogen from soils by leaching or denitrification.
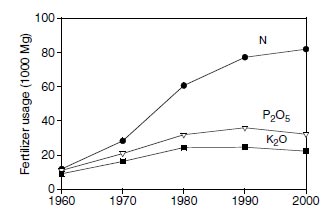 |
| FIGURE 2.3 Worldwide consumption of nitrogen, phosphorus, and potassium in fertilizers for the period 1960–2000. Units of Mg are 1000 kg or one metric ton. (Adapted from http://www.fertilizer.org/ifa/statistics/indicators/tablen.asp) |




