Soil Analysis
A close relationship between the plant-available sulfur content of the soil and yield is a prerequisite for a reliable soil method. Such a significant correlation was verified in pot trials under controlled growth conditions (103,175-178). Several investigations have shown, however, that the relationship between inorganic soil sulfate and crop yield is only weak, or even nonexistent, under field conditions (103,179-181). Such missing or poor correlations are the major reason for the large number of different methods of soil testing, and they justify ongoing research for new methods (114,182-185). Soil analytical methods for plant-available sulfate differ in the preparation of the soil samples, concentration and type of extractant, duration of the extraction procedure, the soil-to-extractant ratio, the conditions of extraction, and the method that is used for the determination of sulfur or sulfate-S in the extract. A serious problem with regard to all laboratory methods is the treatment and preservation of soil samples prior to analysis. Increased temperature and aeration of the sample during storage increase the amount of extractable sulfur by oxidizing labile organic sulfur fractions, and occasionally mobilize reduced inorganic sulfur (186-188).Besides water, potassium or calcium dihydrogenphosphate solutions are the most commonly used solvents to extract plant-available sulfate from soils (189,190). Soils with a high sulfate adsorption capacity are low in pH, so that phosphate-containing extractants extract more sulfate than other salt solutions because of ion-exchange processes. Sodium chloride is also used in countries where soils are frequently analyzed for available nitrate (183,191,192). Less frequently, magnesium chloride (193) or acetate solutions are employed (194,195). Other methodical approaches involve, for instance, anion-exchange resins (196,197) and perfusion systems (198).
In aerated agricultural soils, the organic matter is the soil-inherent storage and backup for buffering sulfate in the soil solution (199-201), and methods are described which focus on capturing organic sulfur fractions that might be mineralized during the vegetation period and thus contribute to the sulfate pool in soils (183,202-204). Such special treatments are, for example, the heating of the samples or employing alkaline conditions or incubation studies, which allow the measurement of either the easily mineralized organic sulfur pool or the rapidly mineralized organic sulfur. Most methods, however, extract easily soluble, plant-available sulfate.
>The practical detection limit of sulfur determined by ICP-AES was 0.5 mg S L-1, corresponding to 3.3 mg S kg-1 (205) in the soil. On sulfur-deficient sites, however, sulfate-S concentrations of only 2 mg S kg-1 were measured regularly in the topsoil by ion chromatography (206). Ion chromatography is much more sensitive, with a practical detection limit of 0.1 mg SO4-S L-1 (corresponding to 0.67 mg S kg-1), allowing sulfate-S to be determined at low concentrations in soils. Additionally, this fact explains why soil sulfate-S measured by ICP-AES is usually below the detection limit. No matter which method is applied, and on which soils or crops the method is used, there is an astonishing agreement in the literature for approximately 10 mg SO4-S kg-1 as the critical value for available sulfur in soils (68,192,207). With the most common methods for the determination of sulfur (ICP and the formation of BaSO4), values of <10 mg S kg-1 will identify a sulfurdeficient soil with a high probability.
As expected, comparisons of different extractants and methods revealed that under the same conditions, all of these methods extract more or less the same amount of sulfate from the soil (178,182,183,185,198,203,207-209). Occasionally observed differences among methods were more likely to be caused by interferences due to the extractant itself (183) rather than by the method of sulfate-S determination (186,187).
As there is virtually no physicochemical interaction between the soil matrix and sulfate, the amount that is present and extractable from the soil is the main indicator commonly used to describe the sulfur nutritional status of a soil. Opinions in the literature on whether or not soil testing is a suitable tool for determining the sulfur status of soils vary from high acceptance (210-215) down to full denial (179,216-220).
Conclusions leading to high acceptance were always drawn from pot trials, which usually yield high correlation coefficients between soil analytical data, and give sulfur content or sulfur uptake of plants as the target value (103,178,183,185,192,194,198,212,221-223,225). Pot trials are always prone to deliver very high correlations between soil, and plant data or yield, as there is no uncontrolled nutrient influx and efflux. However, in the case of field surveys involving a greater range of sites and environmental factors, correlations are poor or fail to reach significance (103,180). For the relationship between available sulfur in soils and foliar sulfur, larger surveys employing a wide range of available sulfur in soils (5 to 250 mg S kg-1), and plants (0.8 to 2.1 g S kg-1), reported correlation coefficients for a total of 1701 wheat and 1870 corn samples of r=0.292 (P≤0.001) and r = 0.398 (P≤0.001), respectively (195). Timmermann and coworkers (225) determined a correlation coefficient of r = 0.396 (P<0.05) for 93 oilseed rape samples. In the field surveys conducted by Schnug (103), a significant relationship could not be verified for 489 oilseed rape samples (r = 0.102, P> 0.05) or for 398 cereal samples (r = 0.098, P> 0.05). These results imply that a maximum of 16% of the variability of the sulfur concentrations in leaves can be explained by the variability of available sulfur in soils. However, Timmermann et al. (225) were able to improve the relationship between soil and plant data by using the ratio of available sulfur and nitrogen in soils (Nmin/Smin) instead of just sulfur. This application gave a value of r = -0.605 (P≤0.01), which still explains less than one third of the variability.
The key problem of soil analysis for plant-available sulfur is that it is a static procedure that aims at reflecting the dynamic transfer of nutrient species among different chemical and biological pools in the soil. This concept is appropriate if the sample covers the total soil volume to which active plant roots have access and if no significant vertical and lateral nutrient fluxes occur to and from this specific volume. Sulfate, however, has an enormously high mobility in soils and can be delivered from sources such as subsoil or shallow groundwater, and sulfur has virtually no buffer fraction in the soil. Thus, the availability of sulfate is a question of the transfer among pools in terms of space and time rather than among biological or chemical reserves. Under field conditions sulfate moves easily in or out of the root zones so that close correlations with the plant sulfur status can hardly be expected. Attempts have been made to take subsoil sulfate into account by increasing the sampling depth (103,226-230), but the rapid vertical and lateral mobility of sulfate influences subsoils too. Thus, this procedure did not yield an improvement of the expressiveness of soil analytical data (103,225).
The soil sulfur cycle is driven by biological and physicochemical processes which affect flora and fauna. The variability of sulfate-S contents in the soil over short distances is caused by the high mobility of sulfate-S. Sulfate is an easily soluble anion, and it follows soil water movements. Significant amounts of adsorbed sulfate are found only in clay and sesquioxide-rich soil horizons with pH values<5, which is far below the usual pH of northern European agricultural soils. Seasonal variations in mineralization, leaching, capillary rise, and plant uptake cause temporal variations in the sulfate-S content of the soil (205). The high spatiotemporal variation of sulfate in soils is the reason for the inadequacy of soil analysis in predicting the nutritional status of sulfur in soils. Thus, under humid conditions, the sulfur status of an agricultural site is difficult to assess (231). An overview of the factors of time and soil depth in relation to the variability of sulfate-S contents is given in Figure 7.13. The highest variability of sulfate-S could be observed on two sites in soil samples collected in April (Figure 7.13). On a sandy soil, the variability was distinctly higher at the second and third dates of sampling in comparison with a loamy soil, but time-dependent changes were significant only in the deeper soil layers. Though the range of sulfate-S contents measured was smaller on the loamy soil than on the sandy soil, the differences proved to be significant in all soil layers between the first and third and second and third dates of sampling respectively (Figure 7.13).
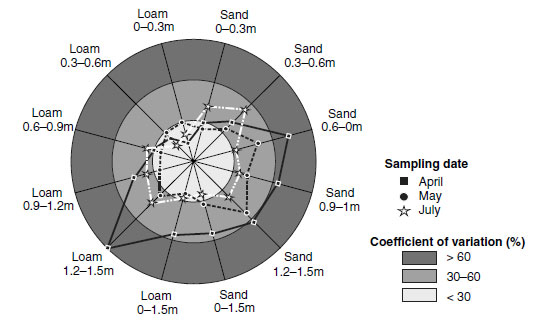 |
| FIGURE 7.13 Spatiotemporal variability of the sulfate contents of different soil layers in two soil types. (From Bloem, E. et al., Commun. Soil Sci. Plant Anal., 32, 1391-1403, 2001.) |
Sources and sinks commonly included in a sulfur balance are inputs by depositions from atmosphere, fertilizers, plant residues, and mineralization, and outputs by losses due to leaching. A frequent problem when establishing such simple sulfur balances is that the budget does not correspond to the actual sulfur supply. The reason is that under temperate conditions it is the spatiotemporal variation of hydrological soil properties that controls the plant-available sulfate-S content. A more promising way to give a prognosis of the sulfur supply is a site-specific sulfur budget, which includes information about geomorphology, texture, climatic data, and crop type and characteristics of the local soil water regime (Figure 7.14).
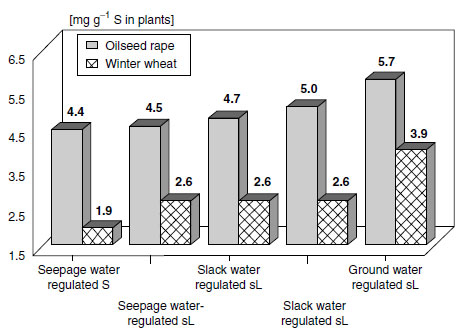 |
| FIGURE 7.14 Total sulfur content of young leaves of oilseed rape and total aboveground material of winter wheat at stem extension in relation to soil hydrological parameters and soil texture (S=Sand; sL=sandy Loam) on the Isle of Ruegen. (From Bloem, E., Schwefel-Bilanz von Agraroekosystemen unter besonderer Beruecksichtigung hydrologischer und bodenphysikalischer Standorteigenschaften, Ph.D. thesis, TU-Braunschweig, Germany, 1998.) |
The results presented in Figure 7.14 reveal that plant sulfur status is distinctly higher on sites with access to groundwater than on sandy soils not influenced by groundwater. The significance of plant-available soil water as a source and storage for sulfur has been disregarded or underestimated so far. However, especially under humid growth conditions, plant-available soil water is the largest contributor to the sulfur balance (205). Leaching and import from subsoil or shallow groundwater sources (184,205) can change the amount of plant-available sulfate within a very short time. Groundwater is a large pool for sulfur, because sulfur concentrations of 5 to 100 mg S L-1 are common in surfaces near groundwater (205,232). There are three ways in which groundwater contributes to the sulfur nutrition of plants. First, there is a direct sulfur input if the groundwater level is only 1 to 2 m below the surface, which is sufficient to cover the sulfur requirement of most crops as plants can utilize the sulfate in the groundwater directly by their root systems. Second, groundwater, which is used for irrigation, can supply up to 100 kg S ha-1 to the crop (205,233-235), but irrigation water will contribute significantly to the sulfur supply only if applied at the start of the main growth period of the crop. Third, the capillary rise of groundwater under conditions of a water-saturation deficit in the upper soil layers leads to a sulfur input. This process is closely related to climatic conditions. The sulfur supply of a crop increases with the amount of plant-available water or shallow groundwater. The higher the water storage capacity of a soil, the less likely are losses of water and sulfate- S by leaching and the greater is the pool of porous water and also the more likely is an enrichment of sulfate just by subsequent evaporation. Thus, heavy soils have a higher charging capacity for sulfate- S than light ones.




