Iron Levels in Plants
Iron Uptake
Transport of iron to plants roots is limited largely by diffusion in the soil solution (41,42), and thus the absorption is highly dependent on root activity and growth, and root length density.The overall processes of iron acquisition by roots have been described in terms of different strategies to cope with iron deficiency (Figure 11.6) (10,43). Strategy 1 plants, such as dicots and other nongraminaceous species, reduce Fe(III) in chelates by a rhizodermis-bound Fe(III)-chelate reductase and take up released Fe2+ ions into the cytoplasm of root cells by a Fe2+ transporter. Strategy 2 plants, mostly grasses, release phytosiderophores that chelate Fe(III) ions and take up the phytosiderophore–Fe(III) complex by a transporter (44,45). A more recently postulated Strategy 3 may involve the uptake of microbial siderophores by higher plants (46), although this could be an indirect use of microbial siderophores through exchange chelation with phytosiderophores in Strategy 2 plants or through FeIII chelate reductase in Strategy 1 plants (47,48).
In Strategy 1 plants, one of the major responses to iron deficiency is the acidification of the rhizosphere, brought about by differential cation-anion uptake (49), the release of dissociable reductants (8,50) and particularly by the action of an iron-deficiency-induced proton pump in the plasmalemma of rhizodermis cells of apical root zones (51). This acidification of the rhizosphere serves to make iron more available and to facilitate the required Fe(III)-chelate reductase activity (52). There is also an enhanced growth of root hairs (53) and the development of structures like transfer cells in the rhizodermis (10) as a response to iron deficiency.
In chickpea (Cicer arietinum L.) subjected to iron deficiency, anion and cation uptake were shown to be depressed, but anion uptake was depressed more than cation uptake (54). This effect gives rise to excess cation uptake, with consequent release of H+ ions in a direct relationship to the extent of the cation-anion imbalance. The origin of the H+ release in such circumstances could be through enhanced PEP carboxylase activity (55).
The release of reductants increases the reduction of Fe3+ to Fe2+ in the apoplast, and has been linked to compounds such as caffeic acid (56,57). These may reduce Fe3+ to Fe2+ ions, and also chelate the ions either for uptake or for reduction on the plasmalemma. Such reduction of Fe3+ on the plasma membrane involves an iron-chelate reductase. It was thought at one time that there are two forms of such reductases, a constitutive form that works at a low capacity and is continuously present, and an inducible form that works with high capacity and is induced under iron deficiency (10). However, in tomato (Lycopersicon esculentum Mill.), iron deficiency gives rise to increased expression of constitutive FeIII-chelate reductase isoforms in the root plasmalemma (58). Action of the FeIII-chelate reductase is the rate-limiting step of iron acquisition of Strategy 1 plants under deficiency conditions (59-61). Genes encoding for proteins in FeIIIchelate reductase and involved with the uptake of Fe2+ in Fe-deficient plants have been identified in the Strategy 1 plant Arabidopsis thaliana, and have been named AtFRO2 and AtIRT1, respectively (62,63).
In Strategy 2 plants the phytosiderophores, nonprotein amino acids such as mugineic acid (64), are released in a diurnal rhythm following onset of iron deficiency (43,52). This release occurs particularly in the apical regions of the seminal and lateral roots (65). The phytosiderophores form stable complexes with Fe3+ ions, and these complexes are taken up by a constitutive transporter in the plasmalemma of root cells (66). Activity of this transporter also increases during iron deficiency. Mutants such as corn (Zea mays L.) ys1/ys1 are very susceptible to iron chlorosis (44).
In the Strategy 1 species cucumber (Cucumis sativus L.), Fe3+ attached to the water-soluble humic fraction is apparently reduced by the plasmalemma reductase, allowing uptake to occur (67,68), whereas in Strategy 2 barley (Hordeum vulgare L.), there is an indirect method for uptake of this Fe3+ component that involves ligand exchange between the humic fraction and phytosiderophores released in response to iron deficiency (68). Uptake of iron then occurs as a Fe(III)-phytosiderophore complex. In Strategy II plants, iron deficiency also leads to a small increase in the capacity to take up Fe2+, uptake previously thought only to occur in Strategy 1 plants (69).
It has been suggested in the past that the large root apoplastic pool of iron could be a source of iron for uptake into plants under iron deficiency. However, the apoplastic pool occurs largely in the older roots (34), yet the mobilization of rhizosphere iron and the uptake mechanisms that are induced under iron deficiency stress occur in the apical zones of the roots, so this seems unlikely (70). The Strategy 1 and Strategy 2 mechanisms are switched on by mild iron deficit stress, although under severe deficiency they become less effective. They are switched off within a day of resumption of iron supply to the plant.
The various iron transporters in plant cells have been well characterized. They include Nramp3 transporters on the tonoplast, and IRT1, IRT2 and Nramp transporters on the plasmalemma (71). Nramp (natural resistance associated macrophage proteins) transporters are involved in metal ion transport in many different organisms, and in Arabidopsis roots, three different Nramps are upregulated under iron deficiency. A model of iron transport in Arabidopsis has been shown elsewhere (72).
The transporter used by Strategy 1 plants is an AtIRT1 transporter, whereas Strategy 2 plants take up the phytosiderophore-Fe(III) complex by ZmYS1 transporters (44,45).
Uptake of zinc, and possibly manganese and copper also, may increase in Strategy 2 plants under iron deficiency, because although the iron-phytosiderophore transporter is specific to iron complexes, the presence of the phytosiderophores in the rhizosphere may increase the availability of these other ions both in the rhizosphere itself and in the apoplast (73).
As well as uptake through roots, iron is able to penetrate plant cuticles, at least at 100% humidity. Chelates of Fe3+ were shown to penetrate cuticular membranes from grey poplar (Populus x Canescens Moench.) leaves without stomata with a half-time of 20 to 30 h (74), although at 90% humidity Fe3+ chelated with lignosulfonic acid was the only chelate tested that still penetrated the membrane. Sachs himself showed that iron is taken up by plants after application to the foliage, and iron chelates have been applied to foliage to correct iron deficiencies because inorganic iron salts are unstable and phytotoxic (see (3)). Fe(III) citrate and iron-dimerum have been found to penetrate the leaves of chlorotic tobacco (Nicotiana tabacum L.) plants, and to be utilized by the cells (75), but it is the chelated forms of iron that enter most effectively.
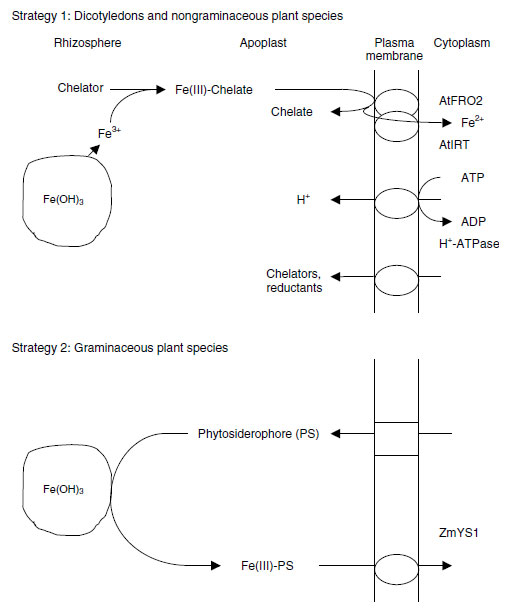 |
| FIGURE 11.6 Strategies for acquisition of Fe in response to Fe deficiency in Strategy 1 and Strategy 2 plants. (Redrawn from R�mheld, V., Schaaf, G., Soil Sci. Plant Nutr., 50:1003-1012, 2004.) |
Movement of Iron within Plants
Once taken up by root cells, iron moves within cells and between cells. The understanding of iron homeostasis at the subcellular level is incomplete, and the role of the vacuole is uncertain. A carrier called AtCCC1 may transport iron into vacuoles, and AtNRAMP3 and AtNRAMP4 are candidates for transporting it out (72). Of the cellular organelles, mitochondria and chloroplasts have a high requirement for iron, and the chloroplasts may be sites of storage of iron (76). Transport into chloroplasts is stimulated by light (77), and it occurs in the Fe(II) form (78).
Knowledge of the movement of iron between cells is also incomplete. Experiments in which 59Fe-labelled iron-phytosiderophores were fed to roots of intact corn plants for periods of up to 2 h demonstrated intensive accumulation of iron in the rhizodermis and the endodermis (72,79). This accumulation was higher with iron deficiency stress, and probably reflected the role of increased number of root hairs and increased expression of the ZmYS1 iron-phytosiderophore transporter.
From the endodermis, the iron is loaded into the pericycle and from there into the xylem. Very little is known about these processes. Once in the shoots, much of the iron is present in the apoplast, from where it is loaded into the cytoplasm and into the organelles where it is required. It was thought at one time that high soil pH would raise shoot apoplastic pH and that this action would make iron unavailable for transport into leaf cells. However, this is not the case, as high root zone HCO3- has been shown not to increase apoplastic pH of leaves in both nutrient-solution-grown sunflower (Helianthus annuus L.) and field-grown grapevine (Vitis vinifera L.) (80), a result that is also in agreement with recent experiments of Kosegarten et al. (81,82). In experiments on grapevine, the presence of bicarbonate in the uptake medium was shown to inhibit uptake of iron and its translocation to the shoots, primarily by inhibiting the Fe(III) reduction capacity of the roots (83). Also, the recently discussed role of nitrate in iron inactivation in leaves and induction of chlorosis due to an assumed increased leaf apoplast pH (82) could not be confirmed (84). Probably, this nitrate-induced chlorosis in solution-cultured sunflower plants is a consequence of an impeded iron acquisition by roots as a consequence of a nitrate-induced pH increase at the uptake sites of the roots.
Movement of iron salts in phloem is obviously possible as Rissm�ller observed retranslocation of iron from senescent leaves of beech trees long ago (3). However, it is usually thought that iron deficiency symptoms occur in young leaves rather than in old leaves because iron is not easily retranslocated in nonsenescent plants. However, such retranslocation is not confined to the senescent leaves of trees, as it has also been observed to occur out of young leaves of Phaseolus vulgaris subjected to iron deficiency (85,86).
Nicotianamine seems to be involved in phloem loading for retranslocation of iron and possibly in phloem unloading and uptake of iron into young leaves and reproductive organs. The maize ZmYS1 protein not only mediates transport of iron-phytosiderophore complexes (87,88), but experiments on this transporter in yeast and Xenopus have shown that it can also transport Fe(II)- nicotianamine and Fe(III)-nicotianamine (88). The AtYSL2 homolog of this protein has been implicated in lateral movement of iron in the vascular system of Arabidopsis thaliana (89,90), and its OsYSL2 homolog in rice has been suggested to be involved in transport of Fe(II)-nicotianamine in phloem loading and translocation of metals into the grain (91). Expression of a nicotianamine synthase gene from Arabidopsis thaliana in Nicotiana tabacum gave increased levels of nicotianamine, more iron in the leaves of adult plants, and improvement in the iron use efficiency of plants grown under iron deficiency stress (92).




