Cell-Specific Expression of Tropane Alkaloid Biosynthetic Genes
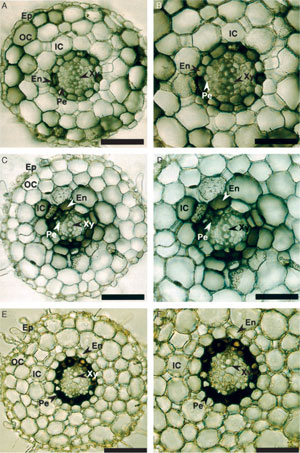
The main site of tropane alkaloid biosynthesis was demonstrated to be in the roots by classical grafting experiments in which scions from tropane alkaloidproducing species were grafted onto rootstock from nonproducing species. The resultant plants did not accumulate alkaloids. The reciprocal graft experiments yielded plants that accumulated alkaloids (cited in Hashimoto et al., 1991). These experimental results suggest that tropane alkaloids are synthesized in roots and transported to aerial parts of the plant. With these early experiments in mind, histochemical localization of pmt, tr-I, tr-II, and h6h has been carried out.tr-I and h6h, specific to scopolamine biosynthesis, and tr-II, specific to calistegin biosynthesis, have been localized to the H. niger root (Hashimoto et al., 1991; Nakajima and Hashimoto, 1999). Accumulation was highest in
lateral roots. tr-I and tr-II accumulated with cell-specific patterns that differed from those of h6h, implying a transport of an alkaloid biosynthetic intermediate that must occur somewhere between tropine and hyoscyamine (Fig. 10.12). Using GUS fusions, A. belladonna pmt promoter activity has been histochemically localized to root pericycle and h6h promoter activity was found both in root pericycle and tapetum and pollen grains (Suzuki et al., 1999a,b) The cell-specific expression of these promoters appears to be species specific (Kanegae et al., 1994).
Once again, multiple cell types are involved in an alkaloid biosynthetic pathway and transport of biosynthetic intermediates must be involved, thereby introducing another possible level of regulation.