Glycerolipids and Fatty Acid Modification
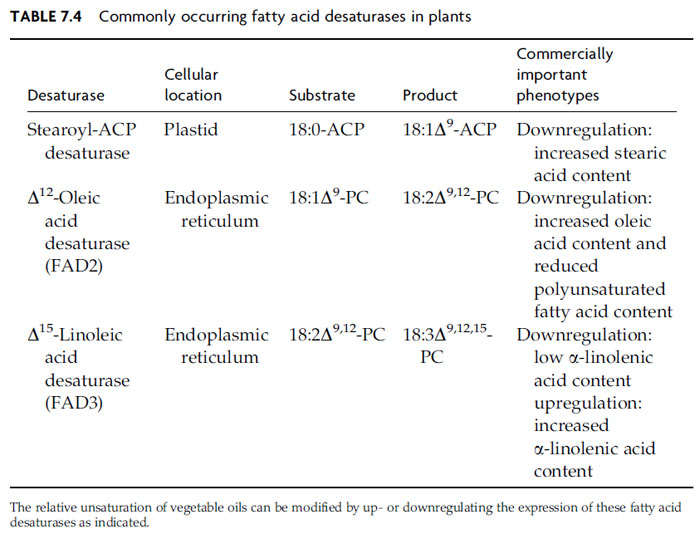
Phosphatidic acid is generally metabolized by one of two enzymes. CDP-DAG synthase, an enzyme found in ER, plastids and mitochondria, generates substrate for production of phosphatidylglycerol, phosphatidylinositol, and phosphatidylserine. The other enzyme, phosphatidate phosphatase, releases DAG, a vital precursor of PC, phosphatidylethanolamine and TAG, as well as sulfolipid and galactolipid. In some plants,microsomal phosphatidate phosphatase suppliesDAG for both plastidial and microsomal glycerolipid synthesis, while in others, separate plastidial and microsomal isoforms contribute. Analysis of the phosphatase is complicated further by isozymes involved in signaling and lipid catabolism. Based on work with developing safflower seeds, Ichihara et al. proposed that an isoform used during oil deposition moves between a cytosolic pool and the ER, depending on cytosolic fatty acid concentrations (Ichihara et al., 1990). This arrangement could allow feedforward regulation of the TAG synthetic pathway initiated by the phosphatase.TAG composition can be radically affected by fatty acid modifications that take place on glycerolipid substrates. As noted earlier, 18:1Δ9 accounts for virtually all of the unsaturated fatty acid exported by a typical plastid. Production of the polyunsaturated fatty acids so common in vegetable oils involves a series of two ER-localized desaturases that act on fatty acids esterified to either sn-position of PC or less prominent phospholipids (Fig. 7.4 and Table 7.4). The first enzyme, typically described as the Δ12-oleic acid desaturase or FAD2, inserts a double bond 12 carbons from the carboxyl end of esterified 18:1Δ9, producing 18:2Δ9,12 (linoleic acid). This enzyme is sometimes referred to as the ω-6 desaturase, which indicates that the double bond is inserted at the sixth carbon atom from the methyl end of the 18:1Δ9 substrate. A more careful analysis showed that this desaturase actually references the site of double-bond insertion based on the position of the Δ9 double bond of its monounsaturated substrate (Schwartzbeck et al., 2001). The second enzyme, the D15-linoleic acid desaturase or FAD3, converts 18:2Δ9,12 to 18:3Δ9,12,15 (a-linolenic acid). As with FAD2, this enzyme is sometimes referred to as the ω-3 desaturase, which indicates that the double bond is inserted at the third carbon atom from the methyl end of its substrate. Engeseth and Stymne found that FAD2 and FAD3 will also desaturate fatty acids that contain hydroxyl and epoxy groups (Engeseth and Stymne, 1996). When determining insertion sites for new double bonds, these enzymes appear to count the unusual functional groups as substitutes for prior double bonds.
 |
Again, the ER enzymes have plastidial counterparts, which act primarily on glycolipid substrates. FAD2 and FAD3 and the analogous plastidial desaturases share eight conserved histidines arranged as H(X3–4)H(X7–41)H(X2–3)HH(X61–189) H(X2–3)HH, and it has been proposed that these histidines are associated with an active site di-iron cluster (Shanklin and Cahoon, 1998). The same motif occurs in enzymes catalyzing a range of fatty acyl desaturation, hydroxylation, and epoxidation reactions (Shanklin and Cahoon, 1998).




