Medium-Chain-Length Polyhydroxyalkanaote
MCL-PHAs are typically described as elastomers, although their actual physical properties are very diverse, ranging from soft plastic to glue and rubber, and are primarilydependent on themonomer composition (deKoning, 1995).Monomers present in MCL-PHA may contain a wide spectrumof functional groups, including unsaturated bonds and halogenated groups (Steinbüchel and Valentin, 1995).There are two main routes for the synthesis of MCL-PHA in bacteria (Fig. 8.6) (Steinbü chel and Fü chtenbusch, 1998; Steinbü chel and Hein, 2001). The first is the synthesis of PHA using intermediates of fatty acid β-oxidation. This pathway is found in several bacteria, such as Pseudomonas oleovorans and Pseudomonas fragii, which can synthesize MCL-PHA from either alkanoic acids or fatty acids. In these bacteria, the monomer composition of the PHA produced is directly influenced by the carbon source added to the growth media. Typically, the PHA is composed of monomers that are 2n (n ≥ 0) carbons shorter than the substrates added to the media. For example, growth of P. oleovorans on octanoate (C8) generates a PHA copolymer containing C8 and C6 monomers, whereas growth on dodecanoate (C12) generates a PHA containing C12, C10, C8, and C6 monomers (Lageveen et al., 1995). Alkanoic acids present in the media are transported into the cell where they are first converted toCoAesters before being directed to the β-oxidation pathway where a number of 3-hydroxyacyl-CoA intermediates can be generated. Since the PHA synthase accepts only the R-isomer of 3-hydroxyacyl- CoA and the bacterial b-oxidation of saturated fatty acids generates only the S-isomer of 3-hydroxyacyl-CoA, bacteria must have enzymes capable of generating R-3-hydroxyacyl-CoA. One potential enzyme is a 3-hydroxyacyl-CoA epimerase, mediating the reversible conversion of the S- and R-isomers of 3-hydroxyacyl-CoA, although no protein or gene encoding such activity has yet been unambiguously identified (Yang et al., 1986). In contrast, monofunctional enoyl-CoA hydratase II enzymes, converting directly enoyl-CoA to R-3-hydroxyacyl-CoA, have been identified in several bacteria, including Aeromonas caviae (Fukui et al., 1998; Reiser et al., 2000; Tsuge et al., 2000). Finally, it is speculated that a 3-ketoacyl-CoA reductase that could specifically generate R-3-hydroxyacyl-CoA may exist in bacteria, although such an enzyme has not yet been unambiguously identified. It has, however, been shown that the enzyme 3-ketoacyl-acyl carrier protein (ACP) reductase, participating normally in the fatty acid biosynthetic pathway, may also act on 3-ketoacyl-CoA to generate R-3-hydroxyacyl-CoA, and thus contribute to MCL-PHA synthesis (Taguchi et al., 1999).
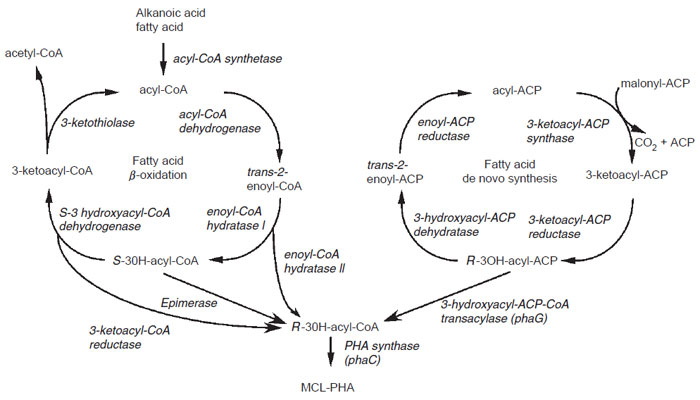 |
| FIGURE 8.6 Pathways for MCL-PHA synthesis. Synthesis of MCL-PHA in bacteria can be accomplished either through the use of intermediates of the fatty acid β-oxidation cycle (left) or of the de novo fatty acid biosynthetic pathway (right). |
The second route for MCL-PHA in bacteria is through the use of intermediates of fatty acid biosynthesis (Fig. 8.6). This pathway is also found in numerous Pseudomonads. In contrast to P. oleovorans and P. fragii, which can synthesize MCL-PHA only from related alkanoic acids present in the growth media, Pseudomonas aeruginosa and Pseudomonas putida can synthesize a similar type of MCL-PHA when grown on unrelated substrates, such as glucose (Huijberts et al., 1992; Steinbüchel and Lü tke-Eversloh, 2003). In these bacteria,MCL-PHAis formed from the 3-hydroxyacyl-ACP intermediates of the de novo fatty acid biosynthetic pathway. phaG is a key enzyme in this pathway, having a 3-hydroxyacyl-CoAACP transferase activity responsible for converting the R-3-hydroxyacyl-ACP intermediate of the fatty acid biosynthetic pathway to R-3-hydroxyacyl-CoA, the substrate for the PHA synthase (Rehm et al., 1998).
Synthesis of MCL-PHA in Plants
The first approach used to synthesize MCL-PHA in plants was to divert the 3-hydroxyacyl-CoA intermediates of the b-oxidation of endogenous fatty acids. Since in plants b-oxidation occurs in the peroxisomes, PHA biosynthetic proteins needed to be targeted to this organelle. The phaC1 synthase from P. aeruginosa was thus modified at the carboxy end by the addition of peroxisomal targeting signal. The modified phaC1 gene was expressed under the control of the CaMV35S promoter and transformed into A. thaliana (Mittendorf et al., 1998). Appropriate targeting of the PHA synthase in plant peroxisomes was demonstrated by immunolocalization. TEM also showed the presence of typical PHA inclusions within the peroxisomes. The monomer composition of the MCL-PHA produced in plants reflected well the broad substrate specificity of the PHA synthase of P. aeruginosa. Thus, peroxisomal PHA was composed of over 14 different monomers, including saturated and unsaturated monomers ranging from 6 to 16 carbons (Mittendorf et al., 1998). The majority of 3-hydroxyacids found in plant MCL-PHA could be clearly linked to the corresponding 3-hydroxyacyl-CoA generated by the b-oxidation of saturated and unsaturated fatty acids. The production of peroxisomal MCL-PHA was relatively low, with a maximal level of 0.4% dwt in 7-day-old germinating seedlings. In leaves, PHA level decreased to ~0.02% dwt. Interestingly, a two- to threefold increase in PHA was observed during leaf senescence. These data support the link between β-oxidation and PHA synthesis, since this pathway, in association with the glyoxylate cycle, is most active during germination and senescence where they are involved in the conversion of fatty acids to carbohydrates. In contrast to PHB synthesis in the cytoplasm and plastid, no negative effects of peroxisomal MCL-PHA accumulation on plant growth or seed germination were observed (Mittendorf et al., 1998).
Similar to the PHA synthase from R. eutropha, the PHA synthase of P. aeruginosa is thought to accept only the R-isomer of 3-hydroxyacyl-CoAs. The wide range of monomers found in plant MCL-PHA suggests that, as with bacteria, plants also have enzymes capable of converting the β-oxidation intermediates S-3-hydroxyacyl- CoA to the R-isomer. Such enzymes could be either the 3-hydroxyacyl-CoA epimerase present on the plant MFP or an enoyl-CoA hydratase II activity that is specific for the generation of R-3-hydroxyacyl-CoA from trans-2-enoyl-CoA. A third route for the synthesis of a narrow range of R-3-hydroxyacyl-CoA is the hydration of cis-2-enoyl-CoA by the enoyl-CoA hydratase I activity of the MFP. The substrate cis-2-enoyl-CoA is derived from the β-oxidation of unsaturated fatty acids having a cis double bond at an even position, such as that found in linoleic and linolenic acids (Poirier, 2002).
Growth of transgenic plants in liquid media supplemented with detergents containing various fatty acids was used to study how to influence the quantity and monomer composition of PHA produced from β-oxidation. Addition of external fatty acids to plants resulted in both an increased accumulation of MCL-PHA and a shift in the monomer composition that reflected the intermediates generated by the β-oxidation of the external fatty acids (Mittendorf et al., 1999). For example, addition of the detergent polyoxyethylenesorbitan esterified to lauric acid (Tween-20) to the media resulted in an eight- to tenfold increase in the amount of PHA synthesized in 14-day-old plants compared to plants growing in the same media without detergent. The monomer composition of the MCLPHA synthesized media containing Tween-20 showed a large increase in the proportion of saturated even-chain monomers with ≤12 carbons, and a corresponding decrease in the proportion of all unsaturated monomers. This shift in monomer composition is accounted by the fact that β-oxidation of lauric acid, a 12 carbon saturated fatty acid, gives saturated 3-hydroxyacyl-CoA intermediates of 12 carbons and lower. Further experiments have shown that addition of either tride-, tridecenoic acid (C13:1 D12), or 8-methyl-nonanoic acid in the plant growth media resulted in the production of MCL-PHA containing mainly saturated odd-chain, unsaturated odd-chain, or branched-chain 3-hydroxyacid monomers, respectively (Mittendorf et al., 1999). These results demonstrated that the plant b-oxidation cycle was capable of generating a large spectrum of monomers that can be included in MCL-PHA even from fatty acids that are not present in significant quantities in plants. Furthermore, ‘‘feeding’’ experiments with these unusual fatty acids demonstrated that all 3-hydroxyacids between 6 and 16 carbons that could be generated by the β-oxidation cycle (via the 3-hydroxyacyl-CoA intermediate) were found in the MCL-PHA. These results supported the concept that the monomer composition of PHA could be used as a tool to study the degradation pathway of fatty acids, including unsaturated fatty acids.
As an alternative to the addition of external fatty acids, modulation of the monomer composition of MCL-PHA synthesized in peroxisomes was also achieved by modifying the endogenous fatty acid biosynthetic pathway (Mittendorf et al., 1999). The first example of this approach was the expression of the peroxisomal PHA synthase in a mutant of A. thaliana deficient in the synthesis of triunsaturated fatty acids. MCL-PHA produced from this mutant was almost completely deficient in all 3-hydroxyacids derived from the degradation of triunsaturated fatty acids, including triunsaturated monomers (Mittendorf et al., 1999). Since numerous fatty acids desaturases have now been cloned and expressed in transgenic plants to control the number and position of unsaturated bonds in fatty acids, this approach could be extended to further modulate the proportion of a number of 3-hydroxyacid monomers in PHAs.
The second approach used to influence the quantity and monomer composition of MCL-PHA was the coexpression of a medium-chain thioesterase in the plastid with a PHA synthase in the peroxisome. Studies on transgenic plants expressing a laurate acyl-ACP thioesterase in the plastid of either leaves or seeds of rape revealed the presence of a futile cycling of lauric acid whereas a substantial portion of the unusual fatty acid was degraded through peroxisomal β-oxidation instead of accumulating in lipids (Eccleston and Ohlrogge, 1998; Eccleston et al., 1996). These studies on lauric acid-producing rapeseed indicated that expression of a thioesterase might be a way of increasing the carbon flux toward β-oxidation and peroxisomal PHA biosynthesis. This hypothesis was tested in A. thaliana by combining the constitutive expression of the peroxisomal PHA synthase with the caproyl-ACP thioesterase from Cuphea lanceolata in the plastid (Mittendorf et al., 1999). Expression of both enzymes led to a seven- to eightfold increase in the amount of MCL-PHA synthesized in plant shoots as compared to transgenics expressing only the PHA synthase. Furthermore, the composition of the MCL-PHA in the thioesterase/PHA synthase double transgenic plant was shifted toward saturated 3-hydroxyacid monomers containing 10 or fewer carbons. This shift is in agreement with an increase in the flux of decanoic acid toward b-oxidation triggered by the expression of the caproyl-ACP thioesterase (Mittendorf et al., 1999). Interestingly, constitutive expression of the related lauroyl-ACP thioesterase in A. thaliana was shown not to lead to an increase in the genes or enzymes involved in β-oxidation (Hooks et al., 1999).
The relation between fatty acid futile cycling and peroxisomal PHA synthesis was further extended to the developing seeds (Poirier et al., 1999). Synthesis of MCL-PHA has been demonstrated in seeds of A. thaliana by expressing the peroxisomal PHA synthase gene under the control of the seed-specific napin promoter. In such transgenic plants, MCL-PHAs accumulated to 0.006% dwt in mature seeds and the monomer composition was relatively similar to the PHA synthesized in germinating seedlings. Expression of both the PHA synthase and caproyl-ACP thioesterase in the leucoplasts of developing seeds resulted in a nearly 20-fold increase in seed PHA, reaching 0.1% dwt in mature seeds. Furthermore, as found with the expression of these two enzymes in whole plants, coexpression in seeds resulted in a large increase in the proportion of 3-hydroxyacid monomers containing 10 or fewer carbons in PHA. These data clearly indicate that even though expression of the caproyl-ACP thioesterase in seeds leads to the accumulation of medium-chain fatty acids in triacylglycerides, there are still a significant proportion of these fatty acids that are channeled toward b-oxidation. This flux toward the β-oxidation cycle is thought to be quite significant, considering that there is only a fourfold difference between the maximal amount of PHA synthesized in germinating seedlings (0.4% dwt), where β-oxidation is thought to be maximal, and the PHA synthesized in the developing seeds expressing the thioesterase (0.1% dwt), where metabolism should be mainly devoted to the synthesis of fatty acid instead of degradation.
Synthesis of MCL-PHAin the peroxisomes of developing seeds has alsodemonstrated the presence of an increased cycling of fatty acids toward β-oxidation in plants deficient in the enzyme diacylglycerol acyltransferase (DAGAT) (Poirier et al., 1999). The tag1 mutant of A. thaliana was shown to be deficient in DAGAT activity in developing seeds, resulting in a decreased accumulation of triacylglycerides and corresponding increase in diacylglycerides and free fatty acids in mature seeds (Katavic et al., 1995). It was hypothesized that the imbalance created between the capacity of the plastid to synthesize fatty acids and the capacity of the lipid biosynthetic machinery of the ER to include these fatty acids into triacylglycerides might have two basic consequences: either fatty acid biosynthesis would be reduced (feedback inhibited) in order to match it with triacylglyceride biosynthesis or excess fatty acids that cannot be included in triacylglycerides would be channeled toward β-oxidation. Expression of the peroxisomal PHAsynthase in the tag1 mutant resulted in a tenfold increase in the amount of MCL-PHA accumulating in mature seeds compared to expression of the transgene in wild-type plants (Poirier et al., 1999). Although these results do not address whether fatty acid biosynthesis is decreased in the tag1 mutant, they nevertheless clearly indicate that a decrease in triacylglyceride biosynthesis results in an increase in the flux of fatty acids toward β-oxidation. Thus, carbon flux to the β-oxidation cycle can be modulated to a great extent and appears to play an important role in lipid homeostasis in plants even in tissues that are primarily devoted to lipid biosynthesis, such as the developing seeds.
Analysis of futile cycling of fatty acids in developing seeds has been extended to transgenic plants accumulating the unusual fatty acids, ricinoleic acid and vernolic acid (Moire et al., 2004). A. thaliana expressing either the Ricinus communis oleate 12-hydroxylase or the Crepis palaestina linoleate 12-epoxygenase under the control of the napin promoter was shown to accumulate approximately twofold more MCL-PHA in developing seeds compared to control. Although relatively small compared to the increase in PHA observed in transgenic plants expressing the C. lanceolata caproyl-ACP thioesterase, the twofold increase in MCL-PHA was quite significant considering that the steady level of either hydroxy or epoxy fatty acids accumulated in transgenic seeds represented only 6.3 mol% or 3.1 mol%, respectively. Thus, clearly, a larger proportion of unusual fatty acids were being degraded via peroxisomal β-oxidation in developing seeds compared to the common fatty acids. Interestingly, microarray analysis of nearly 200 genes involved in fatty acid biosynthesis and degradation, including the genes encoding enzymes of the β-oxidation cycle, revealed no changes in gene expression in transgenic developing seeds expressing either C. lanceolata caproyl-ACP thioesterase, R. communis oleate 12-hydroxylase, or C. palaestina linoleate 12-epoxygenase (Moire et al., 2004). These results indicated that analysis of peroxisomal PHA is a better indicator of the flux of fatty acid through b-oxidation than the expression profile of genes involved in lipid metabolism.
Synthesis of a ‘‘hybrid’’ PHA copolymer has been reported in A. thaliana expressing a PHA synthase from A. caviae modified at the carboxy terminal end for targeting to the peroxisome (Arai et al., 2002). Expression of this PHA synthase under the control of the CaMV35S promoter leads to the accumulation of a PHA containing even-chain and odd-chain monomers ranging from 4 to 6 carbons. The maximal amount of PHA accumulated in leaves and seeds was 0.04% and 0.0032% dwt, respectively. Growth of transgenic plants in media containing Tween-20 increased the total amount of PHA synthesized without affecting appreciably the monomer composition (Arai et al., 2002). The incorporation of ~25 mol% of 3-hydroxyvalerate into PHA raises the interesting question of the source of the odd-chain monomer. Although odd-chain monomers have been detected in MCLPHA synthesized from the expression of the P. aeruginosa PHA synthase in the peroxisome, the amount of odd-chain monomers was very low (<1 mol%). It is possible that an α-oxidation pathway could generate odd-chain intermediate from even-chain fatty acids and that this pathway is more active toward shorter chain intermediates (i.e., 6 carbon fatty acids). Although a gene involved in α-oxidation has been identified, the corresponding protein has not been linked to the peroxisome (Hamberg et al., 1999). Thus, despite evidence of a complete α-oxidation pathway in plants, the link between this pathway and the peroxisome needs to be established. PHA thus offers potentially a unique handle to study α-oxidation in plants.
In the bacterial pathway of MCL-PHA synthesis from intermediates of fatty acid biosynthesis, the enzyme phaG plays a key role, catalyzing the conversion of R-3-hydroxyacyl-ACP to R-3-hydroxyacyl-CoA, the latter being the substrate for the PHA synthase (Rehm et al., 1998). The identification and cloning of the P. putida phaG gene opened the possibility of synthesizing PHA copolymers in the plastids of plants from intermediates of fatty acid biosynthesis. Unfortunately, constitutive expression in the plastid of A. thaliana of only the phaG enzyme led to a marked deleterious effect on plant growth, the plants being dwarfed with crinkly leaves and the seed set being strongly reduced (V. Mittendorf, unpublished results). The reason for this phenotype is not known but is thought to be perhaps due to interference of the transacylase with fatty acid biosynthesis. If this is the case, it would be interesting to know why this does not occur in bacteria expressing phaG. Coexpression in the plastid of the P. aeruginosa PHA synthase along with phaG did not conclusively lead to PHA accumulation in Arabidopsis (V. Mittendorf, unpublished results). Analogous experiments in potato led to similar conclusions, although evidence for the synthesis of a very small amount of a hydrophobic polymer that could be MCL-PHAs was provided (Romano et al., 2005). Thus, despite the obvious advantages of the plastid as a location for the production ofPHB and P(HB-HV), the synthesis in this organelle of phaCopolymer using fatty acid biosynthetic intermediates appears problematic at present.
The synthesis of MCL-PHA in potato cell lines has been demonstrated through expression of the PHA synthase from P. oleovorans in the cytoplasm (Romano et al., 2003). PHA could be detected only after ‘‘feeding’’ the cell lines with 3-hydroxyoctanoic acid, with the PHA containing only the 8 carbon monomer. These results indicate that while no endogenous 3-hydroxyacyl-CoA could be detected in the cytoplasm, an acyl-CoA synthetase activity capable of converting 3-hydroxyoctanoic acid (that originally comes from the external media) to the corresponding 3-hydroxyacyl-CoA was present. The amount of PHA detected reached up to 1% dwt.




