Variant Fatty Acid Desaturases for Metabolic Engineering of Vegetable Oil Composition
Variant acyl-ACP DesaturasesOleic acid (18:1Δ9) is the primary monounsaturated fatty acid of the seed oils of most plants. However, the seed oils of a number of species contain monounsaturated fatty acids with more or fewer than 18 carbon atoms. These fatty acids can also contain a double bond other than at the Δ9 position. Oils that contain large amounts of these novel monounsaturated fatty acids have been of biotechnological interest because they have physical or biological properties that are not found in the oils of major crop plants. For example, petroselinic acid (18:1Δ6), a novel monounsaturated fatty acid, typically comprises >70% of the seed oil of Apiaceae species. Oils that contain very high levels of this fatty acid are solid at room temperature and are less susceptible to digestion by pancreatic lipases than oils enriched in oleic acid. In addition, monounsaturated fatty acids can be oxidatively cleaved to generate dicarboxylic acids for nylon production. The chain lengths of the resulting acids are determined by the position of the double bond. The cleavage of petroselinic acid (18:1Δ6; Fig. 7.3), for example, yields adipic acid, the C6 dicarboxylic acid that is a precursor of nylon 6,6, the world’s most widely manufactured nylon.
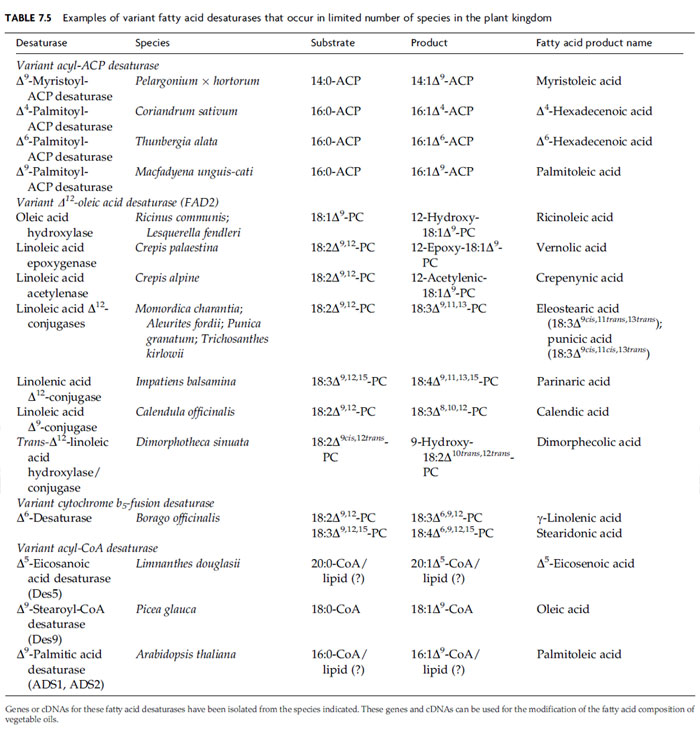
Interestingly, seeds that produce high levels of novel monounsaturated fatty acids with 18 or fewer carbon atoms have been found to contain structurally variant forms of the stearoyl (18:0)-ACP desaturase (Table 7.5) (Shanklin and Cahoon, 1998). Relative to the 18:0-ACP desaturase, these enzymes have altered specificity for the chain length of the acyl-ACP substrate or introduce double bonds at sites other than the D9 position within the fatty acid chain. Variant acyl-ACP desaturases identified to date include a Δ4-palmitoyl (16:0)-ACP desaturase associated with petroselinic acid synthesis in Apiaceae seeds, a Δ6-16:0-ACP desaturase used during the synthesis of D6-hexadecenoic acid (16:1Δ6) by Thunbergia alata seeds, a Δ9–16:0-ACP desaturase linked to production of palmitoleic acid (16:1Δ9) in Macfadyena unguis seeds, and a Δ9-myristoyl (14:0)-ACP desaturase from geranium trichomes that is involved in the synthesis of pest resistant anacardic acids (Cahoon and Ohlrogge, 1994; Cahoon et al., 1994, 1998; Schultz et al., 1996).
 |
Attempts to produce seed oils with novel monounsaturated fatty acids by the transgenic expression of cDNAs for these enzymes have met with only marginal success (Suh et al., 2002). In fact, the case of petroselinic acid highlights the complexity that can be encountered when attempting to engineer novel biosynthetic pathways in oilseed crops. The biosynthesis of this fatty acid requires at least three specialized enzymes: a Δ4-16:0-ACP desaturase, a KAS that efficiently directs the elongation of 16:1Δ4-ACP to 18:1Δ6-ACP, and an acyl-ACP thioesterase that releases 18:1Δ6 from ACP. In addition, biochemical evidence indicates the involvement of a specialized ACP in this pathway, and the sensitivity of the petroselinic acid biosynthesis to salt and detergents in vitro also suggests that this pathway might function as a metabolon or complex of closely associated enzymes. Perhaps as a result of this complexity, the maximum reported level of petroselinic acid accumulation in transgenic A. thaliana seeds is <2% of the total fatty acids (Suh et al., 2002).
A more successful approach for the production of oils with novel monounsaturated fatty acids has resulted from rational modification of the substrate specificity of 18:0-ACP desaturases (Cahoon and Shanklin, 2000; Cahoon and Shanklin, 2000). The availability of a crystal structure for this enzyme has facilitated efforts to redesign its activity (Lindqvist et al., 1996). By modeling the amino acid sequences of variant acyl-ACP desaturases onto the three-dimensional structure of the 18:0-ACP desaturase, it has been possible to convert the 18:0-ACP desaturase into a 16:0-ACP desaturase by substituting only one amino acid at the bottom of the substrate binding pocket. Enzymes rationally designed in this manner have been used to engineer the content of palmitoleic acid (16:1Δ9) and its elongation products to as high as 30% of the total fatty acids of A. thaliana seeds (Cahoon et al., 1997). A complete understanding of how acyl-ACP desaturases position the placement of double bonds in fatty acid chains awaits further structural characterization of these enzymes.
Variant acyl-CoA Desaturases
In contrast to plants, animals and fungi employ a membrane associated fatty acid desaturase known as Δ9-stearoyl (18:0)-CoA desaturase for oleate synthesis. As indicated by this nomenclature, the enzyme is specific for acyl chains bound to coenzyme A, rather than to the ACP employed by soluble 18:0-ACP desaturase in plants. Genes for Δ9–18:0-CoA desaturase-like enzymes have been identified in A. thaliana and other plant species (Shanklin and Cahoon, 1998; Voelker and Kinney, 2001). Two of the Arabidopsis genes ADS1 and ADS2 have been shown to encode ER-localized Δ9 desaturases (Heilmann et al., 2004). A third gene ADS3 encodes a Δ7 desaturase that uses 16:0 bound to the chloroplast lipid monogalatosyldiacylglycerol as its substrate (Heilmann et al., 2004). Interestingly, the ADS1 and ADS2 polypeptides were converted to Δ7 desaturases by targeting of these enzymes to chloroplasts (Heilmann et al., 2004). In addition, a cDNA for a Δ9–18:0-CoA desaturase-like enzyme from white spruce (Picea glauca) seeds has been recently shown to encode a Δ9 desaturase by expression in Saccharomyces cerevisiae (Table 7.5). This enzyme also displays in vitro activity with acyl-CoA substrates (Marillia et al., 2002).
An example of the use of an acyl-CoA desaturase-like enzyme for the metabolic engineering of an oilseed crop resulted from the isolation of genes from seeds of Limnanthes douglasii (Table 7.5) (Cahoon and Shanklin, 2000). The seed oil of this plant contains more than 60% of the unusual monounsaturated fatty acid Δ5-eicosenoic acid (20:1Δ5; Table 7.3). This is an oxidatively stable oil that has superior properties for industrial lubricant applications. The cDNA for this enzyme has been identified among expressed sequence tags from L. douglasii seeds and shown to encode a D5 desaturase by transgenic expression in soybean somatic embryos (Cahoon and Shanklin, 2000). Although this enzyme has significant activity with 16:0 and 18:0 in vivo, it appears to be most active with eicosanoic acid (20:0). In fact, a 20:1Δ5 content of 12% has been achieved in soybean embryos coexpressing the Δ5 desaturase cDNA and a cDNA for an L. douglasii 3-ketoacyl- CoA synthase (KCS) that initiates the elongation of 16:0-CoA to 20:0-CoA. This level of novel monounsaturated fatty acid accumulation exceeds that achieved in all previous attempts to produce such fatty acids through the transgenic expression of naturally occurring variant acyl-ACP desaturases. It is presumed that the L. douglasii Δ5 desaturase uses an acyl-CoA substrate, based on results from in vitro assays of Limnanthes seed preparations (Moreau et al., 1981). However, it cannot be ruled out that the substrate for the L. douglasii desaturase and other acyl-CoA desaturase-like enzymes in plants is, in fact, a fatty acid bound to a lipid, as is the case for FAD2 and FAD3.
Variant Cytochrome b5-fusion Fatty Acid Desaturases
The most successful efforts to introduce biosynthetic pathways for novel unsaturated fatty acids in oilseed crops have involved the production of g-linolenic acid (GLA; 18:3Δ6,9,12; Table 7.3) by transgenic expression of divergent forms of the sphingolipid-8-desaturase. GLA comprises up to 25% of the seed oil of many species of the families including borage (Borago officinalis). This fatty acid is a precursor of prostaglandins and leukotrienes, and oils enriched in GLA have a number of demonstrated therapeutic properties (Barre, 2001). As a result, GLA-enriched oils from seeds of plants such as borage are produced commercially as nutraceuticals. A cDNA for the Δ6 desaturase associated with GLA synthesis in plants has been isolated from developing borage seeds by polymerase chain reaction (PCR) with degenerate oligonucleotides based on partially conserved amino acid sequences in membrane-associated fatty desaturases (Sayanova et al., 1997). This cDNA encodes a polypeptide distantly related to FAD2 and FAD3 as well as to the functionally equivalent Δ6 desaturase from Synechocystis (Table 7.5) (Reddy and Thomas, 1996; Shanklin and Cahoon, 1998). The borage polypeptide is predicted to contain not only the histidine residues characteristic of membrane-type fatty acid desaturases, but also an N-terminal cytochrome b5 domain. Presumably this domain functions as the electron donor for the desaturase portion of the polypeptide, as cytochrome b5 is a cofactor for ER-localized desaturases (Sayanova et al., 1997). Transgenic expression of this cDNA in tobacco leaves resulted in the production of GLA via the Δ6 desaturation of linoleic acid (Fig. 7.4), as well as stearidonic acid (18:4Δ6,9,12,15) comprising up to 10% of total leaf fatty acids (Sayanova et al., 1997). The latter unusual polyunsaturated fatty acid arises either from the Δ15 desaturation of GLA by FAD3 or from the desaturation of α-linolenic acid by the D6 desaturase. Stearidonic acid is found only in trace amounts in borage seeds due apparently to the lack of an active FAD3 in this tissue. Since the discovery of the borage enzyme, fatty acid desaturase-like polypeptides with close structural relationships to the Δ6 desaturase, including the presence of the N-terminal cytochrome b5 domain, have been shown to occur widely in plants (Sperling et al., 1998). These enzymes are sphingolipid- 8-desaturases associated with the synthesis of unsaturated sphingolipid long-chain bases such as sphingenine. They introduce double bonds at the sixth carbon atom from the C-2 position of long-chain base substrates (Sperling et al., 1998).
A borage Δ6 desaturase cDNA was expressed in soybean seeds under control of a strong seed-specific promoter. In the most promising transgenic line, GLA and stearidonic acid comprised 33% and 4%, respectively, of the soybean seed oil (Sato et al., 2004). By comparison, the GLA content of borage oil is typically 20–25% of the total fatty acids. This is perhaps the only case to date in which the accumulation of a novel fatty acid in a transgenic seed oil exceeds that found in the naturally occurring seed oil.
Structural and functional homologues of the borage Δ6 desaturase have been identified in other plant species as well as a wide range of organisms including the moss Physcomitrella patens; animals including Caenorhabditis elegans, mouse and human; and fungal species including Mortierella alpina and Pythium irregulare (Das et al., 2000; Garcı´a-Maroto et al., 2002; Girke et al., 1998; Hong et al., 2002; Napier et al., 2003). The M. alpina and P. irregulare genes have been used to engineer the production of GLA in seed oils of Brassica species (Das et al., 2000; Hong et al., 2002).
The family of cytochrome b5-fusion desaturases has subsequently been found to include Δ4 and Δ5 desaturases, both of which are structurally related to the Δ6 desaturase (Napier et al., 2003). Though these enzymes occur in numerous organisms, neither fatty acid desaturase has yet been identified in plants. Genes for Δ5 desaturases have been found in mammals, C. elegans, and the fungus M. alpina (Knutzon et al., 1998; Michaelson et al., 1998; Napier et al., 2003). This enzyme catalyzes the introduction of the D5 double bond of arachidonic acid (ARA), a C20 polyunsaturated fatty acid. Δ4 Desaturase genes have been isolated from Euglena gracilis and Thraustochytrium sp. and are associated with the formation of the Δ4 double bond of docosahexaenoic acid (DHA), a C22 polyunsaturated fatty acid (Meyer et al., 2003; Qiu et al., 2001a). Collectively, the cytochrome b5–fusion desaturases have been referred to as ‘‘front-end’’ desaturases, based on their catalytic ability to position the site of double bond insertion relative to the carboxyl or ‘‘front end’’ of fatty acid substrates. Similar regiospecificity is displayed by acyl-CoA-type desaturases and soluble acyl-ACP desaturases (Shanklin and Cahoon, 1998). ‘‘Front-end’’ desaturase genes have considerable biotechnological significance for the metabolic engineering of health-promoting, very long-chain polyunsaturated fatty acids in seed oils of transgenic crops.
Variant FAD2s
The typical FAD2 catalyzes the synthesis of linoleic acid by the Δ12 desaturation of oleic acid (Table 7.4). In addition to this widely occurring enzyme, structurally divergent forms of FAD2 have been shown to function in the biosynthesis of unusual fatty acids in seeds of diverse plant species (Fig. 7.4 and Table 7.5) (Voelker and Kinney, 2001). These divergent FAD2 enzymes are of biotechnological interest because they introduce functional groups, such as hydroxy residues and epoxy rings, onto fatty acid chains. The functionalized fatty acids can be used for a variety of industrial applications. Divergent FAD2 genes encode hydroxylases, acetylenases, and some epoxygenases, all enzymes requiring oxygen and employing similar histidine motifs and di-iron clusters (Lee et al., 1998; van de Loo et al., 1995). Relatively small modifications can alter the activity of these enzymes. For example, it has been possible to interconvert Δ12-oleic acid desaturase and Δ12-hydroxylase activities by altering only four to six amino acids (Broun et al., 1998). The substrate in this case is the same, phospholipid-esterified 18:1Δ9. Other members of the family modify preexisting double bonds. Given the right enzyme, the Δ12 double bond of linoleic acid (18:2Δ9,12) may be converted either to a triple bond by Δ12-acetylenase, or to an epoxide ring by the related epoxygenase from C. palaestina (Cahoon et al., 2003; Lee et al., 1998). It should be noted that not all plants produce epoxy-fatty acids via a FAD2-related enzyme. A cytochrome P450 enzyme from Euphorbia lagascae12 double bonds (Cahoon et al., 2002).
In addition, variant forms of FAD2 known as fatty acid conjugases, because they catalyze the formation of conjugated double bond systems, have been identified in seeds of a number of plant species. These enzymes use linoleic acid or a-linolenic acid as substrates. One class of fatty acid conjugase, which is found in seeds of Calendula officinalis, converts the cis-Δ9 double bond into the two adjacent trans-Δ8 and trans-Δ10 double bonds (Cahoon et al., 2001; Qiu et al., 2001b). A second class of conjugase converts the cis-Δ12 double bond into adjacent trans-Δ11 and trans-Δ13 or trans-Δ11 and cis-Δ13 double bonds. The second class of fatty acid conjugases is functional in seeds of species such as Impatiens balsamina, Momordica charantia, Aleurites fordii, and Punica granatum (Cahoon et al., 1999; Dyer et al., 2002; Hornung et al., 2002). Unlike the typical Δ12 desaturase which abstracts hydrogens from the C-12 and C-13 atoms of the oleic acid substrate, the mechanism of fatty acid conjugases is believed to involve the removal of a hydrogen atom from the two carbon atoms that flank an existing double bond. This mechanism has been referred to as a ‘‘‘‘1,4-desaturation’’ (Reed et al., 2002).’’ Fatty acid conjugase cDNAs from M. charantia and C. officinalis have been engineered into somatic embryos of soybean to generate oils with 15–20%-conjugated trienoic (or triunsaturated) fatty acids (Cahoon et al., 1999, 2001). Oils enriched in these fatty acids are useful as quick drying agents in coating materials, such as paint and ink, as a result of the oxidative instability of the conjugated fatty acids.




