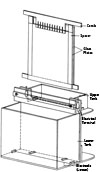
Polyacrylamide-sodium dodecyl sulphate slab gel electrophoresis (SDS-PAGE) of proteins
Polyacrylamide gels are formed by polymerizing acrylamide with a cross-linking agent (bisacrylamide) in presence of a catalyst (persulphate ion) and chain indicator (TEMED; N,N,N’,N’ – tetramethylethylene diamine). Solution are normally degassed by evacuation prior to polymerization since oxygen inhibits polymerization. The porosity of the gel is determinedly the relative proportion of acrylamide monomer to bisacrylamide.
Principle
SDS is an anionic detergent which binds strongly to, and denatures, proteins. The number of SDS molecules bound to a polypeptide chain is approximately half the number of amino acid residues in that chain. The protein-SDS complex carries net negative charges, hence move towards the anode and the separation is based on the size of the protein.Materials
| Stock Acrylamide Solution | |
| Acrylamide 30% | 30g |
| Bisacrylamide 0.8% | 0.8g |
| Water to | 100mL |
| Separation Gel Buffer | |
| 1.875M Tris-HCl | 22.7g pH 8.8 |
| Water to | 100mL |
| Stacking Gel Buffer | |
| 0.6M Tris-HCl | 26g pH 6.8 |
| Water to | 100mL |
| Polymerizing Agents | |
| a) Ammonium persulphate 5% | 0.5g/10mL, prepare freshly before use |
| b) TEMED | fresh from the refrigerator |
| Electrode Buffer | |
| 0.05M Tris | 12g |
| 0.192M Glycine | 28.8g pH 8.2-8.4 |
| 0.1% SDS | 2g No adjustment required |
| Water to | 2mLmay be used 2-3 times |
| Sample Buffer (5X concentration) | |
| Tris-HCl buffer pH 6.8 | 5mL |
| SDS | 5g |
| Sucrose | 5g |
| Mercaptoethanol | 25mL |
| Bromophenol Blue (0.5% W/V solution in water) | 1mL |
| Water to | 10mL |
| Store frozen in small aliquots. Dilute to 1x concentration and use. | |
| Sodium dodecyl sulphate 10% solution – store at room temperature. | |
| Standard Marker Proteins | |
| Protein | MW Daltons |
| a-Lactalbumin | 14,200 |
| Trypsin inhibitor soyabean | 20,100 |
| Trypsinogen | 24,000 |
| Carbonic anhydrase | 29,000 |
| Glyceraldehyde-3-phosphate dehydrogenase, rabbit | 36,000 |
| Albumin, egg | 45,000 |
| Albumin bovine | 66,000 |
| Dissolve the above proteins in single strength sample buffer at a concentration each of 1mg per mL. Load the well with 25-50mL. | |
| Protein Stain Solution | |
| Coomassie brilliant blue R 250 | 0.1g |
| Methanol | 40mL |
| Acetic acid | 10mL |
| Water | 50mL |
| First, dissolve the dye in methanol and proceed. Use fresh preparation every time. | |
| Destainer | |
| As above without the dye. | |
Procedure
- Thoroughly clean and dry the glass plates and spacers, then assemble them property. Hold the assembly together with bulldog chips. Clamp in an upright position. White petroleum jelly or 2% agar (melted in a boiling water bath) is then applied around the edges of the spacers to hold them in place and seal the chamber between the glass plates.
- Prepare a sufficient volume of separating gel mixture (30mL for a chamber of about (18 x 9 x 0.1cm) by mixing the following.
for 15% gel for 10% gel Stock acrylamide solution 20mL 13.3mL Tris-HCl (pH 8.8) 8mL 8mL Water 11.4mL 18.1mL Degas on a water pump for 3 – 5min and then add: Ammonium persulphate solution 0.2mL 0.2mL 10% SDS 0.4mL 0.4mL TEMED 20mL 20mL - Mix gently and carefully, pour the gel solution in the chamber between the glass plates. Layer distilled water on top of the gel and leave to set for 30-60min.
- Prepare stacking gel (4%) by mixing the following solutions (total volume 10mL)
Stock acrylamide solution = 1.35mL
Tris-HCl (pH 6.8) = 1mL
Water = 7.5mL
Degas as above, and then add:
Ammonium persulphate solution (5%) = 50mL
10% SDS = 0.1mL
TEMED = 10mL
Remove the water from the top of the gel and wash with a little stacking gel solution. Pour the stacking gel mixture, place the comb in the stacking gel and allow the gel to set (30-60min). - After the stacking gel has polymerized, remove the comb without distorting the shapes well. Carefully install the gel after removing the clips, agar etc. in the electrophoresis apparatus. Fill it with electrode buffer and remove any trapped air bubbles at the bottom of the gel. Connect the cathode at the top and turn on the DC-power briefly to check the electrical circuit. The electrode buffer and the plates can be kept cooled using a suitable facility so that heat generated during the run is dissipated and does not affect the gel and resolution.
- Prepare samples for electrophoresis, following suitable extraction procedure. Adjust the protein concentration in each sample using the 5-strenght sample buffer and water in such a way that the same amount of protein is present per unit volume. Again the concentration should be such as to given a sufficient amount of protein (50-200mg) in a volume (25-50mL) not greater than the size of the sample well. As general practice, heat sample solution in boiling water for 2-3min to ensure complete interaction between protein and SDS.
- Cool the sample solution and take up the required volume in a microsyringe and carefully inject it into a sample well through the electrode buffer. Making the position of well on the glass plate with a marker pen and the presence of bromophenol blue in the sample buffer facilitate easy loading of the samples. Similarly, load a few well with standard marker protein in the sample buffer.
- Turn on the current to 10-15mA for initial 10-15min until the samples travel through the stacking gel. The stacking gel helps concentration of the samples. Then continue the run at 30mA until the bromophenol blue reaches the bottom of the gel (about 3h). however, the gel may be run at a high current 960-70mA) for short period (1h) with proper cooling.
- After the run is complete, carefully remove the gel from between the plates and immerse in staining solution for at least 3h or overnight with uniform shaking. The proteins absorb the coomassie brilliant blue.
- Transfer the gel to a suitable container with at least 200-300mL destaining solution and shake gently continuously. Dye that is not bound to proteins is thus removed. Change the destainer frequently, particularly during initial periods, until the background of the gel is colorless. The proteins fractionated into band are seen colored blue. As the proteins of minute qualities are stained faintly, destaining process should be stopped at appropriate stage to visualize as many bands as possible. The gel can now be photographed or stored in polythene bags or dried in vacuo for a permanent record (for details see ‘Flurography of polyacrylamide gels’).
Notes
- All chemicals and distilled water should be of high quality. The solutions prepared should be filtered before use. The solutions can be stored refrigerated for 1-2 weeks; aged solution result in poor resolution of proteins.
- Acrylamide as a monomer is highly neurotoxic; handle with extreme care.
- Prefer to use the gel immediately following polymerization although the separation gel after setting can be stored overnight by wetting with four-fold diluted separation gel buffer or with stacking gel and comb placed over it to avoid drying.
- Degassing of gel mix should be adequate for easy polymerization.
- During polymerization of the gel, heat is evolved.
- The water layered over the separation gel should be completely removed for quick polymerization of the stacking gel.
- Some troubles and remedies are as follows:
Trouble Cause Remedy a. Failure or slow polymerization of the gel Presence of oxygen
Absence of catalysts
Stock aged
Glass platesDegas the solution sufficiently
Check if all solution mixed
Use fresh solutions
Degrease the plates with ethanolb. Poor sample wells Stacking gel and comb Fit and/or remove the comb carefully. c. Long duration of the run Air-bubbles interference Flush air-bubbles d. Staining is poor The dye absorption is not efficient The dye may be old, hence use a strong solution of the dye or change to a more sensitive stain The staining is patchy Solid dye Dissolve the dye completely or filter The stained bands are decolorized The dye is removed excessively Restain the gel and stop destaining appropriately e. Protein bands are inadequately resolved Insufficient electrophoresis Run for longer time Separation gel Change the percentage of gel f. Protein bands are wavy Excess persulphate Use optimum concentration of presulphate Bands have become streaked Proteins remain aggregated, denatured or insoluble Use fresh sample buffer or extra SDS or centrifuge the sample extract sufficiently g. Protein dye migration is not even Gel is partly insulated by air bubbles. Remove air bubbles before electrophoresis Insufficient cooling Improve the cooling or run at a low current h. The protein band-lane broadens at the bottom of the separation gel Sample density Load equal volume of samples in each well, equal strength sample buffer, leave no empty wells in the middle Sample diffuses while loading the wells Low density of sample Increase the concentration of sucrose or glycerol in the sample buffer - Handle the polyacrylamide gel carefully to avoid any breakage.
- The slab gel along the glass plates is placed vertically in the electrophoresis tank and run. It is therefore called ‘vertical slab gel electrophoresis’.
- In 10% polyacrylamide gels, the low molecular weight (~10,000 daltons) polypeptides will migrate diffused; for fine resolution of these polypeptides use gels of higher (15%) acrylamide concentration.
- Any band of 0.1mg protein is visualized by coomassie brilliant blue staining in SDS-PAGE; for visualizing proteins of lower concentration below 0.1mg high sensitive (silver staining) method is prepared.
References
1. Laemmli, U K (1970) Nature 227, 680.2. Manual on Techniques in Molecular Biology (Proteins) (1986). Workshop held at the Department of Biochemistry Tamil Nadu Agri Univ Coimbatore p 16.