Ascorbic Acid Oxidase
(L Ascorbate: Oxygen oxidoreductase EC 1.10.3.3)Ascorbic acid oxidase is widespread in plant tissues. The role of this enzyme is to regulate the levels of oxidized and reduced glutathione and NADPH as shown below.
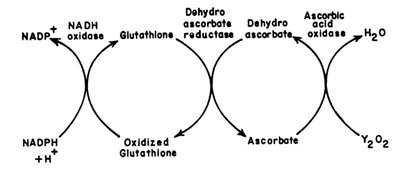 |
| The activity of this enzyme is increased during infection. |
Principle
The method used for assay of ascorbic acid oxidase depends on the fact that within a certain range of enzyme concentrations, the rate of oxygen consumption during ascorbic acid oxidation is proportional to the amount of enzyme present. Ascorbic acid has an absorption maximum at 265nm. Hence, the decrease in the absorption peak of the acid due to oxidation by ascorbic acid oxidase is followed spectrophotometrically.
Materials
» Phosphate buffers 0.1M (pH 5.6 and 6.5 separately)» Substrate Solution
Dissolve 8.8mg ascorbic acid in 300mL phosphate buffer (pH 5.6)
» Enzyme Extract
Macerate one part of plant tissue with five parts (w/v) of 0.1M phosphate buffer (pH 6.5) in a homogenizer. Centrifuge the homogenate at 3,000g for 15 min. Use the supernatant as enzyme source. All procedures are carried out at 0-5°C.
Procedure
| 1. |
Pipette out 3mL of substrate solution to each of sample and reference cuvettes of a spectrophotometer. |
| 2. |
Add 0.1mL of enzyme extract to the reference cuvette. (Enzyme is added so as to get a positive increase in absorbance values.) |
| 3. |
Measure the absorbance change at 265nm in 30sec intervals for 5min. |
Calculation
From the linear phase of reaction, compute the change in absorbance per min.Alternatively activity may be expressed in enzyme units. One enzyme unit is equivalent to 0.405mmole oxygen*per min. Ascorbic acid requires only one atom of oxygen per mole oxidized. Therefore, 0.81mmole of 1/2 oxygen per min is utilized per enzyme unit. Ascorbic acid has an E1cm1%of 760 at 265nm. From this it is calculated that ascorbic acid has an absorbance of 4.4 per mmole in a 3mL volume.
Hence, one enzyme unit (0.81mmole 1/2 oxygen per min) would be equivalent to an absorbance change of 3.58 per min.




