Isolation of Animal Material (Tissue)
Disaggregation of tissue
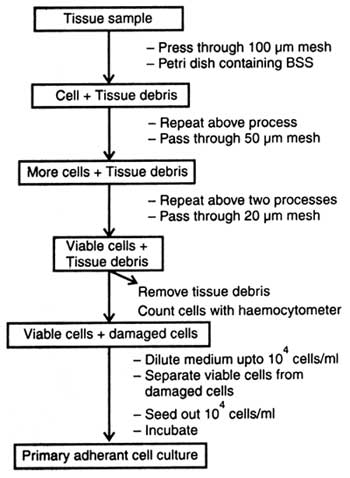
Some of tissues consist of cells which are tightly aggregated. Tissue like epithelium is impregnated with Ca2+ and Mg2++ ions that provide integrity to it. Therefore, for getting primary culture it is necessary that tissue must be disaggregated either mechanically or using enzymes or chemicals so that cell suspension could be obtained. The cells in suspension grow to produce primary culture.
The viable dissociated cells are now termed as 'primary cells'. When the primary cells are seeded on culture medium in high density, these grow well. Thus the primary viable cells of primary culture are called adherant culture and the cells adherant cells. Moreover, at this stage some of the non-viable cells if growing along with adherant cells can be separated out by using the second medium. Similarly, primary culture can also be grown in suspension. In suspension the non-viable cells be removed from primary disaggregates by centrifugation using Ficoll and sodium metrizoate. In this way viable cells are separated from non-viable cells.
(ii) Enzymatic disaggregation. Enzymes are also used for dislodging the cells of tissue. By using enzymes a high number of cells is obtained. Moreover, in embryonic tissue a high number of undifferentiated cells with least extracellular matrix is found. Therefore, disaggregation of embryonic tissue occurs more readily than that of adult or new borns. In addition, in fragile tissue such as tumours the chances of cell death and cell recovery are more than the normal tissues. There are two important enzymes used in tissue disaggregation, collagenase and trypsin.
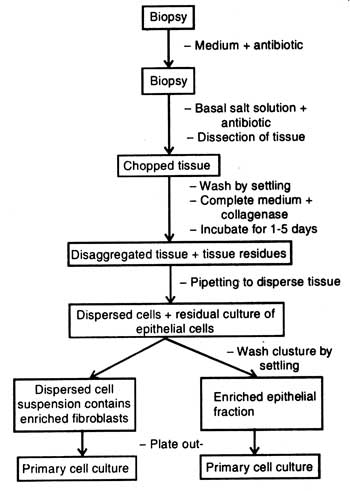
The crude collagenase also contains non-specific proteases. First, the biopsy tissues are kept in medium containing antibiotics. Thereafter, the tissue to be disaggregated is dissected into pieces in basal salt solution containing antibiotics (Fig. 6.2). The chopped tissue is properly washed with sterile distilled water and transferred in complete medium containing collagenase. After five days of treatment the mixture is pipetted so that the medium may get dispersed.
When the whole treatment is left for some times, the residual clusture of epithelial cells settles on bottom of test tubes. Clustures present in test tubes are washed by settling or the dispersed cell suspension is made free from the enzyme collagenase by centrifugation. Suspension consists of enriched fibroblast fraction which is plated out on medium. Similarly, the clusture which is washed by settling consists of enriched epithelial fraction. It is also plated out on medium.
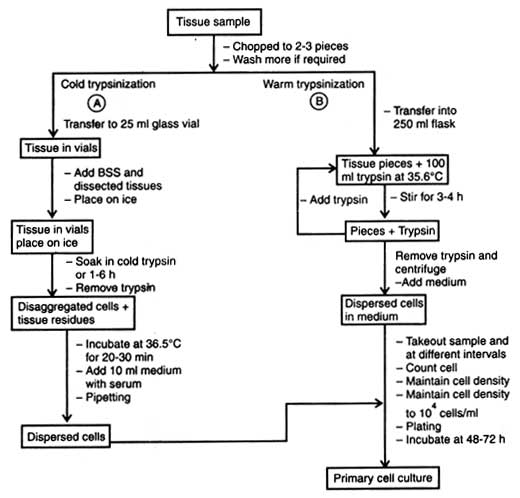
(b) Use of trypsin. Use of trypsin for disaggregation of tissue is called trypsinization. However, the enzyme trypsin in crude form is commonly used for embryonic tissue because many kinds of cell can tolerate it and different types of tissues are significantly affected. Besides, serum or trypsin inhibitors (e.g. soybean trypsin inhibitor) can neutralize its residual enzyme activity only in serum-free medium. On the basis of role of temperature on trypsin, activity is of two types, cold trypsinization and warm trypsinization.
Warm trypsinization. Similar to cold trypsinization, the tissue sample is chopped into 2-3 pieces (Fig. 6.2B) and washed in distilled water keeping in glass vial. The pieces are transferred into 250 ml flask containing 100 ml warm trypsin (36.5°C).
After different trypsinization time, samples are pipetted, cells counted using haemocytometer and cell density maintained to 104cells/ml. The cells are plated on medium and incubated for 48-72 hours for cell growth.