Transgenic Animals
Much has been described about the methods of transfer of genes/DN A fragments/chromosomes into animal cells in different sections of biotechnology in this website either through microinjection, drug delivery system, electroporation or mediated by viruses. Cell manipulation has been aimed at production of novel chemicals, pharmaceutical drugs or improvement in animal breed. The other purpose has been to study the structure and function of genes through molecular markers and genome mapping (see precedent section). Improvement in livestock has already given good results for increased milk production in catties, increased growth rate of livestock and fish, production of valuable proteins in large quantities in milk, urine and blood of livestock, wool production in transgenic sheep.
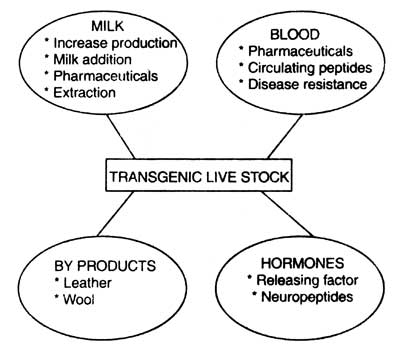
Thus the transgenic animals can be used as bioreactors for large scale production of valuable recombinant chemicals such as hormones, interferons, proteins, etc. Thus, manufacturing of recombinant drugs through transgenic animals is called 'molecular farming' or 'molecular pharming'. Areas of possible investigation of domestic livestock by using the transgene technology has been given in Fig. 7.7 (Ebert, 1989).
Strategies for Gene Transfer
Desired foreign genes are transferred into animal cells/embryos via virus, microinjection, targeted gene transfer methods, etc. as discussed below:
Transfection of animal cells/embryos
During 1970s, attempts were made to produce transgenic animals. The first success was achieved in 1976 when mouse embryos were infected by retrovirus. The retroviruses are supposed to be an efficient vector that transfect the animal cells and deliver its genes leading to production of transgenic cells/animals. The other viruses used for this purpose are vaccinia virus, adeno-associated virus, herpes virus and bovine papiloma virus (see Gene Therapy). For detailed description of retroviruses, their morphological nature and genome, see A Text Book of Microbiology by Dubey and Maheshwari (1999).
The transfected cultured mammalian cells have been used for diagnostics of oncogene (i.e. cancerous gene) as well as for gene therapy. The steps for detection of cancer gene follow : (i) isolation of DNA from tumour cell line, (ii) DNA fragementation into 30-50 kb long pieces through mechanical shearing, (iii) dissolution in phosphate buffer followed by precipitation by adding CaCl2, (iv) pouring of this solution onto a layer of mouse 3T3 cells; foci of cells developed, and (v) use of transfected cells in detection of cancer causing genes.
The other application of transfected cells is in gene therapy. Genes of desired function are inserted in cultured cells. The later is placed in patient's body to rectify the malfunctioning gene.
Transfer through microinjection
Microinjection method has also been developed and variously used in production of transgenic animals. So far gene transfer has been successfully carried out in several classes of animals viz., fish, birds, insects, mammals, etc. (for detail see Genetic Engineering for Human Welfare).
The targeted gene transfer
The other approach is the targeted gene transfer that involves transfer of genes at homologous sites in the host genome. It is done just to replace the wild type of mutant genes. For the first time it was done in bacteria and yeast. In 1985 success has been achieved in human also where human b-globin gene was transferred into recipient cell through recombination.
Targeted gene transfer is possible because the homologous DNA sequences arc present at the targeted site as well as in vector that carries the desired gene of foreign origin. Besides, marker genes are also used to select the cells in which gene has been transferred at targeted site. It is achieved by (i) using marker genes for antibiotic resistance, (ii) hypoxanthine phosphoribosyl transferase (HPRT), and (Hi) polymerase chain reaction.
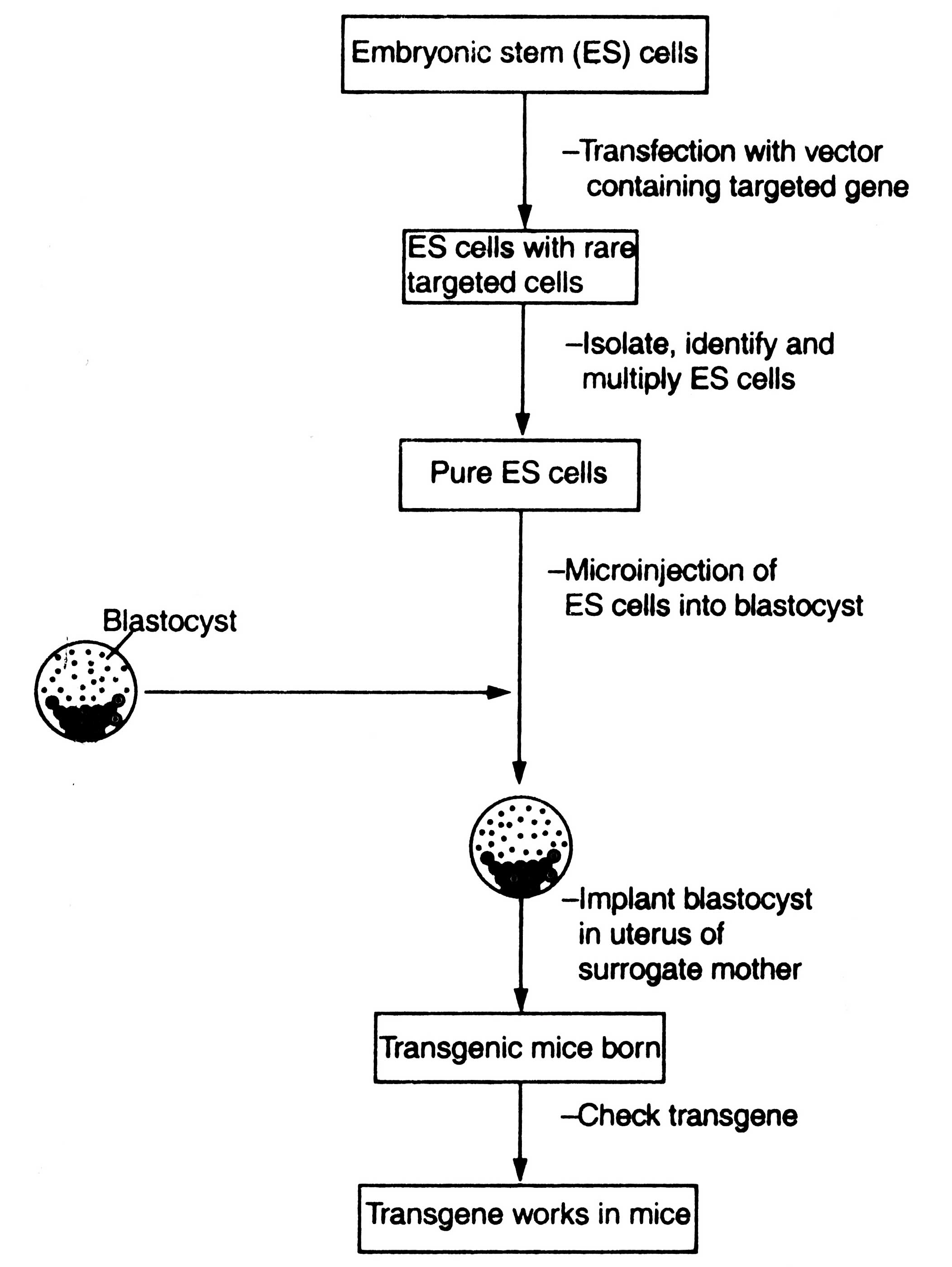
Gene targeting is also done by using embryonic stem (ES) cells as described earlier. The ES cells are allowed to get transfected by vector containing desirable genes. In transfected cells targeting of gene to specific site by homologous recombination occur. Thereafter, the transfected cells are identified and isolated from bulk. They are multiplied and introduced into blastocyst through microinjection. The blastocyst is transferred into uterus of a surrogate mother for further developmental stages. The animals, which are born, are checked for the presence of transgene. The transgenic animal is crossed with a normal one to study the inheritance of introduced foreign gene. An outline of production of transgenic mice is shown in Fig. 7.8.
Transgenic Mammals
For the first time in 1982, there appeared a report on the transfer of human growth hormone gene of rat fused to the promoter of mouse metallothioneine I gene (Palmiter et al 1982, 1983). It was done by microinjection method. As a result of presence of a novel gene, there has been a drastic increase in body weight of mice (for detail method see Genetic Engineering for Human Welfare). Since then a large number of transgenic mammals and other animals have been produced such as cow, pig, rabbit, goats, sheep, fish, etc. The purpose of production of transgenic animals has been to produce more protein in milk and meat, disease resistance, leaner meat, good quality wool and more specifically improvement in genetic traits. They are also used as bioreactor for molecular farming (see Animal bioreactor and molecular farming).
Transgenic Sheep
So far it is not clear why the rate of transgenesis in sheep is very low i.e. 0.1 to 0.2 per cent. It needs improvement by regular check through the biotechnological methods (using PCR, etc). Method of production of transgenic sheep is the same as described for transgenic mice (Fig. 7.8). Due to commercial appeal, the Pharmaceutical Proteins Ltd, Cambridge (UK) provided fund to J.P. Simons for the production of transgenic sheep. In 1988, Simons reported first production of transgenic sheep. He first produced two transgenic ewe that consisted of about 10 copies of human antihaemophilic factor IX gene in the form of cDNA. It was fused with about 10.5 kb long b-lactoglobulin (BLG) gene. Moreover, the BLG gene is important for the expression of gene in mammary gland. The ewes secreted human alpha-1 antitrypsin (ha- 1AT) i.e. human factor IX in milk because the gene had tissue specific expression. Inspite of low expression of transgene, the ha-lAT is active. The transgenic ewes were born in summer 1986. Again they were mated at the end of the year 1986. Single lamb was born from each ewe in 1987 that inherited BLG-factor IX transgene. Due to the presence of both the genes factor IX was secreted in milk.
In 1991, Alan Colman and coworkers at Edinburgh produced five transgenic sheep, four female and one male. The transgene was ovine-b-lactoglobulin promoter fused to ha-1AT gene. The concentration of ha-1AT in milk was recorded to about 35 grams per liter. The biological activity of protein derived from milk was the same as that of plasma derived antitrypsin.
Transgenic Fish
Fish are the important source of fat and proteins for humans and the delicious diets are prepared in certain societies. Therefore, demand of good quality fish is increasing gradually. Gene transfer in embryos of several species of fish such as medaka fish, salmon, carp, zebrafish, goldfish, trout and cattlefish has been successfully achieved. The novel desired gene is introduced into early embryo through microinjection because pronuclei are not easily visible. In some fish transgene is microinjected into the nucleus of oocyte. The introduced gene replicates at the time of development of embryo. In most of the fish fertilization is accomplished out side in water. Therefore, embryo of different developmental stages can be collected easily. In those cases where microinjection technique is not successful, transgenes are incorporated into embryos through electroporation technique.
Inheritance of transgene occurs in the Mendalian way. Stuart et al. (1988) found that spermatozoa of transgenic trout and zebrafish containing foreign DNA transmitted the transgene to the next generation. Normal oocyte fertilized with sperms of transgene fish gave rise to offsprings of which 10-50 per cent of Fl offsprings contained foreign genes. The number of transgenic fish increased in F2 generation.
Animal Bioreactors and Molecular Farming
Transgenic animals are used as bioreactor for mass production of drugs and proteins called molecular farming (pharming). It is a new industry. The Transgenic Scien (an American Company) has produced transgenic mice which secreted in milk about 0.5 grams/liter of hGH. Due to expression of transgene, this mice hormone has no adverse effect. Adverse effect of cattle and pig derived human hormones have been observed. In addition, the pigs that secrete extra hormone, hGH are sterile. This company has started scaling up of production of hGH through transgenic rabbit because of high concentration of protein in milk and short gestation period of rabbit.
A transgenic lamb has been produced by the Institute of Animal Physiology and Genetic Research, Edinburgh (UK). This lamb contains alpha-antitrypsin (aAT) gene. In human the deficiency of aAT causes fluid accumulation in lungs resulting in death of patients. The aAT inhibits the enzyme elastase that digests foreign particles and clears the lungs. In the absence of aAT elastase is not inhibited and, therefore, it digests lung tissue. In lactating milk of transgenic lamb, presence of aAT can be tested. Transgenic sheep can also be used for production of aAT commercially.
Thus the transgenic animals can be used as bioreactors for large scale production of valuable recombinant chemicals such as hormones, interferons, proteins, etc. Thus, manufacturing of recombinant drugs through transgenic animals is called 'molecular farming' or 'molecular pharming'. Areas of possible investigation of domestic livestock by using the transgene technology has been given in Fig. 7.7 (Ebert, 1989).
Strategies for Gene Transfer
Desired foreign genes are transferred into animal cells/embryos via virus, microinjection, targeted gene transfer methods, etc. as discussed below:

Fig. 7.7. Areas of investigation of domestic livestock via transgenic technology (based on Ebert, 1989).
Transfection of animal cells/embryos
During 1970s, attempts were made to produce transgenic animals. The first success was achieved in 1976 when mouse embryos were infected by retrovirus. The retroviruses are supposed to be an efficient vector that transfect the animal cells and deliver its genes leading to production of transgenic cells/animals. The other viruses used for this purpose are vaccinia virus, adeno-associated virus, herpes virus and bovine papiloma virus (see Gene Therapy). For detailed description of retroviruses, their morphological nature and genome, see A Text Book of Microbiology by Dubey and Maheshwari (1999).
The other application of transfected cells is in gene therapy. Genes of desired function are inserted in cultured cells. The later is placed in patient's body to rectify the malfunctioning gene.
Transfer through microinjection
Microinjection method has also been developed and variously used in production of transgenic animals. So far gene transfer has been successfully carried out in several classes of animals viz., fish, birds, insects, mammals, etc. (for detail see Genetic Engineering for Human Welfare).
The other approach is the targeted gene transfer that involves transfer of genes at homologous sites in the host genome. It is done just to replace the wild type of mutant genes. For the first time it was done in bacteria and yeast. In 1985 success has been achieved in human also where human b-globin gene was transferred into recipient cell through recombination.
Targeted gene transfer is possible because the homologous DNA sequences arc present at the targeted site as well as in vector that carries the desired gene of foreign origin. Besides, marker genes are also used to select the cells in which gene has been transferred at targeted site. It is achieved by (i) using marker genes for antibiotic resistance, (ii) hypoxanthine phosphoribosyl transferase (HPRT), and (Hi) polymerase chain reaction.
Transgenic Mammals
For the first time in 1982, there appeared a report on the transfer of human growth hormone gene of rat fused to the promoter of mouse metallothioneine I gene (Palmiter et al 1982, 1983). It was done by microinjection method. As a result of presence of a novel gene, there has been a drastic increase in body weight of mice (for detail method see Genetic Engineering for Human Welfare). Since then a large number of transgenic mammals and other animals have been produced such as cow, pig, rabbit, goats, sheep, fish, etc. The purpose of production of transgenic animals has been to produce more protein in milk and meat, disease resistance, leaner meat, good quality wool and more specifically improvement in genetic traits. They are also used as bioreactor for molecular farming (see Animal bioreactor and molecular farming).
So far it is not clear why the rate of transgenesis in sheep is very low i.e. 0.1 to 0.2 per cent. It needs improvement by regular check through the biotechnological methods (using PCR, etc). Method of production of transgenic sheep is the same as described for transgenic mice (Fig. 7.8). Due to commercial appeal, the Pharmaceutical Proteins Ltd, Cambridge (UK) provided fund to J.P. Simons for the production of transgenic sheep. In 1988, Simons reported first production of transgenic sheep. He first produced two transgenic ewe that consisted of about 10 copies of human antihaemophilic factor IX gene in the form of cDNA. It was fused with about 10.5 kb long b-lactoglobulin (BLG) gene. Moreover, the BLG gene is important for the expression of gene in mammary gland. The ewes secreted human alpha-1 antitrypsin (ha- 1AT) i.e. human factor IX in milk because the gene had tissue specific expression. Inspite of low expression of transgene, the ha-lAT is active. The transgenic ewes were born in summer 1986. Again they were mated at the end of the year 1986. Single lamb was born from each ewe in 1987 that inherited BLG-factor IX transgene. Due to the presence of both the genes factor IX was secreted in milk.
Transgenic Fish
Fish are the important source of fat and proteins for humans and the delicious diets are prepared in certain societies. Therefore, demand of good quality fish is increasing gradually. Gene transfer in embryos of several species of fish such as medaka fish, salmon, carp, zebrafish, goldfish, trout and cattlefish has been successfully achieved. The novel desired gene is introduced into early embryo through microinjection because pronuclei are not easily visible. In some fish transgene is microinjected into the nucleus of oocyte. The introduced gene replicates at the time of development of embryo. In most of the fish fertilization is accomplished out side in water. Therefore, embryo of different developmental stages can be collected easily. In those cases where microinjection technique is not successful, transgenes are incorporated into embryos through electroporation technique.
Animal Bioreactors and Molecular Farming
Transgenic animals are used as bioreactor for mass production of drugs and proteins called molecular farming (pharming). It is a new industry. The Transgenic Scien (an American Company) has produced transgenic mice which secreted in milk about 0.5 grams/liter of hGH. Due to expression of transgene, this mice hormone has no adverse effect. Adverse effect of cattle and pig derived human hormones have been observed. In addition, the pigs that secrete extra hormone, hGH are sterile. This company has started scaling up of production of hGH through transgenic rabbit because of high concentration of protein in milk and short gestation period of rabbit.