Applications in Industries
Products (Secondary metabolites) from Cell Cultures
Plant cells cultured in vitro have been considered to potential source of specific secondary metabolites. Cell cultures may contribute in at least four major ways to the production of natural products. These are as : (i) a new route of synthesis to established products e.g. codeine, quinine, pyrethroids, (ii) a route of synthesis to a novel product from plants difficult to grow or establish e.g. thebain from Papaver bracteatum, (iii) a source of novel chemicals in their own right e.g. rutacultin from culture of Ruta, (iv) as biotransformation systems either on their own or as part of a larger chemical process e.g. digoxin synthesis (Fowler, 1983). Natural products of plants and their associated industries are given in Table 9.1.
Table 9.1. Natural products from plants and their associated industries.
| Plant products | Plant species | Industry | Industrial uses |
| Codeine (alkaloid) | Papaver sominifera | Pharmaceutical | Analgesic |
| Diosgenin (steroid) | Dioscorea deltoidea | " | Antifertility agent |
| Quinine (alkaloid) | Cinchona leitgeriana | " | Antimalaria |
| Digoxin (glycoside) | Digitalis lanata | " | Cardiactonic |
| Scopolamine | Dhatura stramonium | " | Antihypersensitive (alkaloid) |
| Vincristine (alkaloid) | Catharanthus roseus | " | Antileukaemic |
| Pyrethrin | Chrysanthemum cinerariaefolium | Agrochemical | Insecticide |
| Quinine (alkaloid) | C. ledgeriana | Food & drink | Bittering agent |
| Jasmine | Jasmium sp. | Cosmetics | Perfume |
| Saffron | Crocus sativa | Food | Flavoring/coloring agent |
| Taxol | Taxus brevifolia | Pharmaceutical | Ovarian and breast cancer |
Recently, in vitro production of high amount of useful compounds has increased with the success obtained so far in experimental studies. It is hoped that in near future, the industrial production of such compounds by using these techniques would be possible.
Cell suspension and biotransformation Biotransformation is a process through which functional groups of organic compounds are modified by living cells. Biotransformation done by plant cell culture system can be desirable when a given reaction is unique to a plant cell and the product of reaction has a high market value.
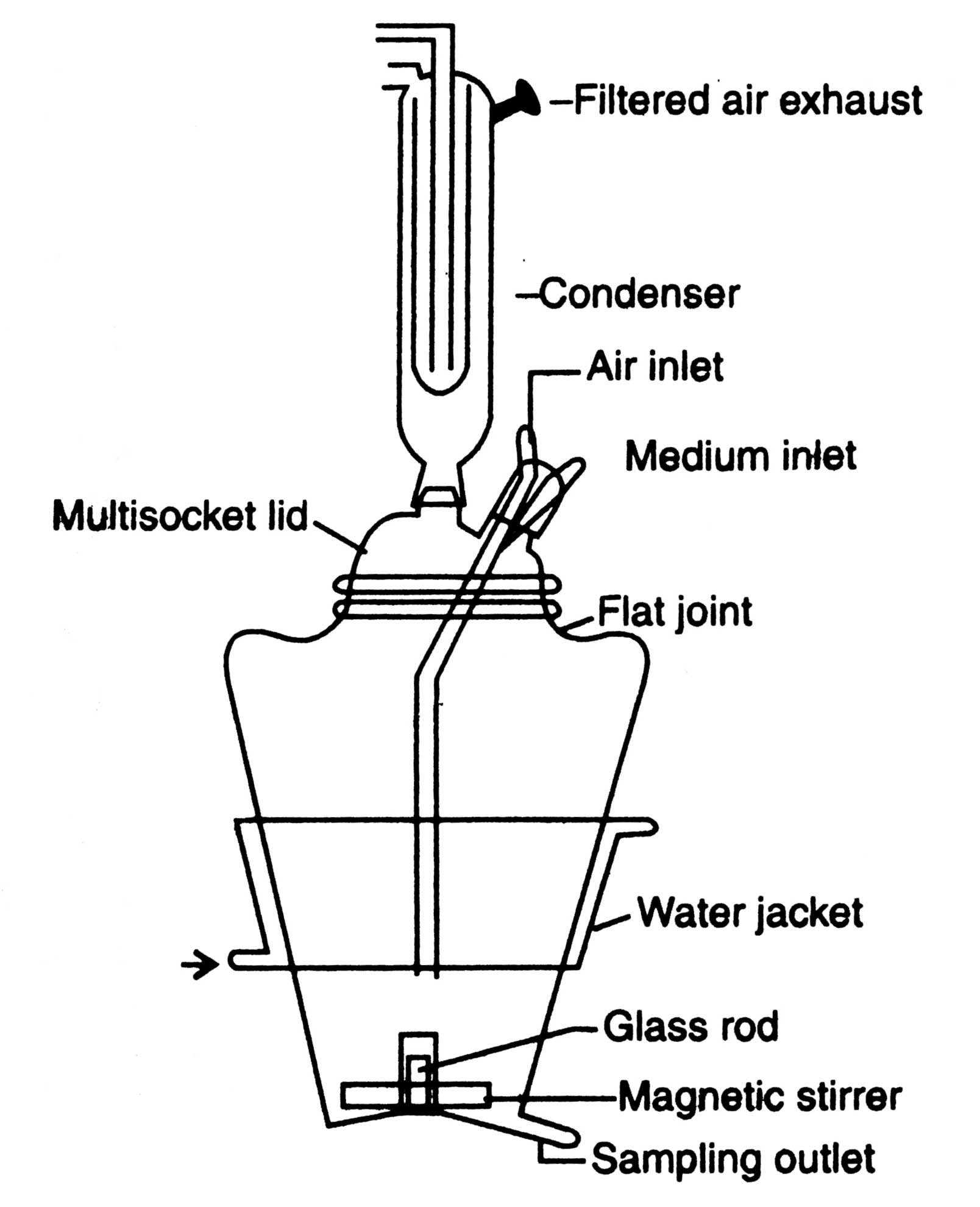
Plant cells are used in a number of ways for biotransformation purposes. The most basic procedure is to supply the cell suspension with the components that is to be transformed, and harvest the products from the culture medium after incubation at suitable conditions. A V-fermenter is used for bio-transformation (Fig. 9.5). Plant cell culture has potential for bioconversion of flavonoids (tannins, anthraquinone), mevalonales, phenyl propanoids (steroids, cardiac glycerides) and alkaloids.
Therefore, it is necessary that comparison between the yield from the higher plant and those from cell culture must be done (Table 9.2). Improvement in product yield through cell culture should be made. This is particularly important in terms of process development and scale up. Cell suspensions are more amenable to scale up from a biochemical engineering standpoint that requires simple bioreactors as compared with more organized tissue system.
Table 9.2. Cell cultures accumulating alkaloids in higher amount than their mother plants.
| Plants | Alkaloids | Yield (% dry weight) | |
| Cell culture | Whole plant | ||
| Ailanthus | Canthin 6-ones | 1:27 | 0.01 |
| Berberis | Jatrorrhizine | 10,0 | 2.0 |
| Catharanthus | Ajmalicine, serpentine | 1.3 |
0.26 |
| Ephedra | Pseudoephidrine | 2.25 | 0.6 |
| Macleaya | Protopine | 9.4 | 0.32 |
| Nicotiana | Nicotine | 3.4 | 2.5 |
| Siephania | Biscoclaurines | 2.29 | 0.92 |
The useful natural products are synthesized through secondary metabolism, hence they are also known as secondary metabolites (for microbial secondary metabolites see Secondary Metabolites).The secondary metabolites include alkaloids, terpenoids, tannins, glycosides (steroids and phenolics) and saponins. Their chief applications are in Pharmaceuticals, in food flavoring and perfumery (Collins and Watts, 1983). According to one estimate (Whitaker and Evans, 1987) 4300 different flavor compounds have been identified in food. Certain flavors consist of one or few related compounds such as 2-isobutylthiazole (tomato flavors), methyl-ethyl cinnamates (strawberry), methyl anthranilate (grape), benzaldehyde (cherry), menthol (mint), safranal (saffron) (Whitaker and Evans, 1987).
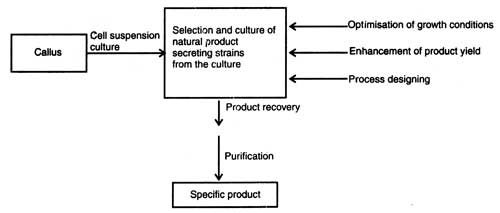
Various secondary metablites that have been produced through cell culture are listed in Table 9.1; design of procedures in biotechnical process of cell cultures and products recovery are shown in Fig. 9.6.
Table 9.3. Secondary metabolites produced through cell suspension culture.
| Plant species |
Secondary metabolites |
| Acer pseudoplatanus | Flavonols and phenolics |
| Catharanthus roseus | Serpentine |
| Daucus carota | Anthocyanin |
| Datura stramonium | Tropane |
| Lithospermum erythrorhizon | Shikonin |
| Mentha Canadensis | Menthol, terpenes |
| M. piperata | Menthol |
| Morinda citrifolia | Anthraquinones |
| Nicotiana tabacum | Tobacco alkaloids, quinone |
| N. rustica | Nicotine |
| Panax ginseng | Saponines |
| Populus nigra | Anthocyanins |
| Rosa gallica | Essential oil |
| Ruta graveolens | Furnanocoumarins |
| Scopolia japonica | Peptides |
| Solanum lacinalium | Solasaline |
| Vinca minor | Indole alkaloids |
Factors affecting product yield
There are a number of factors that affect the selecting high-yielding cell lines, some of them are discussed herewith
(i) Tissue origin genetic character. Plant cells are genetically totipotent, therefore, proper environmental conditions should be given so that any cell may be induced to produce any substance according to the characteristic of parental plants. However, it has been found that low yielding plants produce high contents of products and vice versa). Therefore, such plant parts should be chosen where there is the highest concentration of desired product.
(iii) Selection and screening. Cell clones from better strains are selected which is rather a difficult task. The more difficult work is to detect the very small amount of desired product present in single cell or small population of cells. To reach the goal mutagenic techniques as a selection pressure are applied to develop high yielding cell lines. Radioimmunoassay (RIA) technique has been applied with much success for the screening of a variety of cultures and products. Moreover, the enzyme linked immunosorbent assay (ELISA) has also been used for screening of products (Fowler, 1983).
Large scale yield of secondary metabolites from cultured plant cells can be increased simply by changing the physiological and biochemical conditions from growth medium. But one of the methods of production on increased rate is the use of immobilized plant cells. The method of immobilized plant cells has been found very effective for the production of secondary metabolites as it provides a stable and uniform environment. The plant cells are immobilized in inert matrix and bathed in a medium which does not allow the cell division but keeps the cells in viable conditions for a long time. This obviate the need of further subculturing. There are two commonly! used methods for immobilization : (i) immobilization of cells or subcellular organelles (entrapment in some matrix such as alginate, polyacrylamide, and collagen, or in combination of gels), and (ii) adsorption to an inert substrate such as glass beeds. Examples of cell immobilization are given in Table 9.4.
Table 9.4. Plant cells immobilized by two methods.
| Source of cells | Immobilized substrates | Methods |
| Catharanthus roseus | Alginate, agarose,polyacrylamide, carrageenan,alginate and gelatin | Immobilized ininert substratum |
| Digitalis lanata | Alginate | do |
| Morinda citifolia | Alginate | do |
Different support systems and viability and biosynthetic performance of cells within them have been widely worked out. Calcium alginate is frequently used as support. The enclosing matrix can be contained in a more rigid framework to form a column. Beads are packed into a column or maintained in suspension as free beads or the original alginate cell matrix can be added to an inert support e.g. nylon mesh. These conditions are sufficient to allow the cells to transform the precursors into secondary metabolites.
Future of Plant Tissue Culture Industry in India
There are over hundred companies globally, each capable of producing more than a million plants per annum through micropropagation. Each year the number of such companies and tissue cultured products is increasing. More than 10 export-oriented units for the mass propagation of tissue cultured plantlets of flowering and ornamental plants have been licensed.
In India commercialization of plant tissue cultured seeds was started at a small scale by A.V. Thoman & Co. (AVT) at Manalaroo (Kerala). By using small scale technology developed by NCL and AVT perfected the process and created a super variety of cardamom. This variety could be cropped in two years instead of the usual tree. The yield increased from 70 to 250 kg per hectare, and earning also increased accordingly from Rs. 6,000 to Rs. 25,000 per hectare. Another Indian Company, ITC Agro Tech has developed sunflower hybrid named PAC3425 which has been found to have 11% enhancement in seed production and 26% increase in oil yield as compared to the best quality cultivars.
An lndo-American Hybrid Seeds (IAHS), a Bangalore based company, has started work on plant biotechnology. In 1991, IAHS exported flowers worth 1.4 crore to Holland, U.K. and Denmark. It has introduced better strains of cardamom and banana plants. Tissue cultured banana plants begin to bear fruits in 9 months as compared to 15 months by usual variety. The Southern Petroleum Chemical Industries Corporation (SPIC) has planned to increase the export of tissue cultured ornamental plants viz., carnation, lilies, and chrysanthemum to Holland, Australia and Europe.