Phosphorylation of CTD of a subunit of Pol II
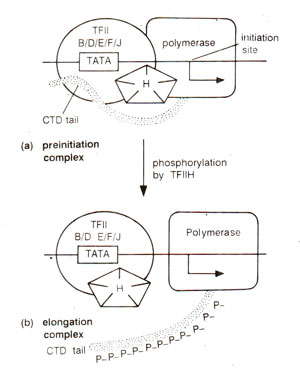
Fig. 32.18. A model showing the action of transcription factor TFIIH (a kinase) on the carboxy terminal domain (CTD) tail of the largest subunits of RNA . polymerase II (note that the polymerase can elongate the RNA on DNA template,, without the transcription factors, once it binds to promoter and gets phosphorylated).
Extensive phosphorylation takes place in the carboxy-terminal domain (CTD) 'tail' of the largest subunit of RNA polymerase II. TFIIH has been shown to be the CTD kinase responsible for phosphorylation of CTD. The kinase is held in check and its activity is increased with the assembly of pre-initiation complex, and with the addition of TFIIE. TFIIH actually consists of five subunits and carries out activities other than phosphorylation of Pol II. In particular, the interaction of TFIIH, TFIIE and TFIIJ may be important for transcription.

Fig. 32.18. A model showing the action of transcription factor TFIIH (a kinase) on the carboxy terminal domain (CTD) tail of the largest subunits of RNA . polymerase II (note that the polymerase can elongate the RNA on DNA template,, without the transcription factors, once it binds to promoter and gets phosphorylated).




