Binding of AA-tRNA at site 'A' of ribosome
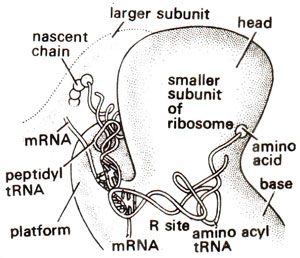
Fig. 34.7. Binding of amino acyl tRNA to R-site, when its anticodon is bound to the next unread codon (redrawn from Sci. Amr., Vol. 345, 1981).
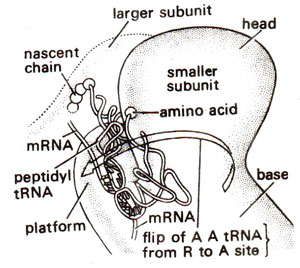
Fig. 34.8. Amino acyl tRNA flips into a position to help its binding on 'A' site (redrawn from Sci. Amer., Vol. 345, 1981).

Fig. 34.9. Formation of EF-Tu-GTP-tRNA ternary complex, which helps entry into 'A' site, and the release of EF-Tu-GDP, which forms Tu-Ts.
Another site called 'R' site located on smaller subunit of ribosome, has also been proposed. The presence of 'R' site has been confirmed and its role has been discussed. It is proposed that 'R' site plays a role in the improvement of accuracy of translation. It is postulated that aminoacyl tRNA first binds to R site involving codon-anticodon pairing (Fig. 34.7). Later aminoacyl tRNA is flipped to A site using energy from GTP molecule (Fig. 34.8). During this flipping, tRNA is held only by codon-anticodon pairing. If this pairing is weak due to mismatching of wrong tRNA, aminoacyl tRNA will fly off the ribosome. R site thus provides a correcting measure.
After formation of 70S initiation complex, the first amino acyl tRNA (AA-tRNA) enters 'A' site. Factors responsible for this entry include the elongation factors EF-Tu (u means unstable when heated) and EF-Ts (s means stable when heated). Although AA-tRNA can bind to ribosome even without the help of elongation factors (EF-Tu, EF-Ts), proper binding occurs only in the presence of EF-Tu, which is responsible for placing AA-tRNA on the A site.
The elongation factor EF-Tu first combines with GTP and changes to an active binary complex (EF-Tu-GTP) which binds with AA-tRNA (but it can not bind to f-met-tRNAfmet) to form a ternary complex (Fig. 34.9).

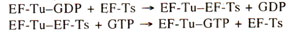
In eukaryotes as typified by red blood cells, there are two elongation factors eEFl and eEF2, the former being considered analogous to EF-T (both EF-Tu and EF-Ts) of E. coli and the latter analogous to EF-G (see later in the section). However, eEFl may have several polypeptides in addition to the two which may be analogous to EF-Tu and EF-Ts. The elongation factors in chloroplasts and mitochondria may be completely independent and have not been characterized so far.

Fig. 34.7. Binding of amino acyl tRNA to R-site, when its anticodon is bound to the next unread codon (redrawn from Sci. Amr., Vol. 345, 1981).

Fig. 34.8. Amino acyl tRNA flips into a position to help its binding on 'A' site (redrawn from Sci. Amer., Vol. 345, 1981).

Fig. 34.9. Formation of EF-Tu-GTP-tRNA ternary complex, which helps entry into 'A' site, and the release of EF-Tu-GDP, which forms Tu-Ts.
This is a catalytic reaction during which a peptide bond is formed between the free carboxyl group (-COOH) of the peptidyl tRNA at the 'P' site and the free amino (-NH2) group present with amino acyl tRNA at the 'A' site. The enzyme involved in this reaction is peptidyl transferase and is an integral part of 50S ribosomal subunit. After the formation of peptide bond, the tRNA at 'P' site is deacylated and the tRNA at 'A' site now carries the polypeptide.
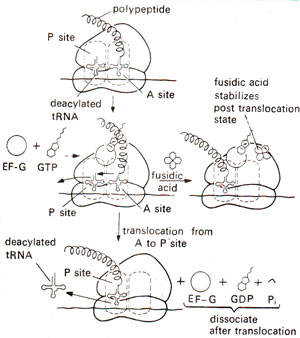
Fig. 34.10. Translocation of peptidyl tRNA from 'A' site to 'P' site with the help of elongation factor EF-G and GTP.
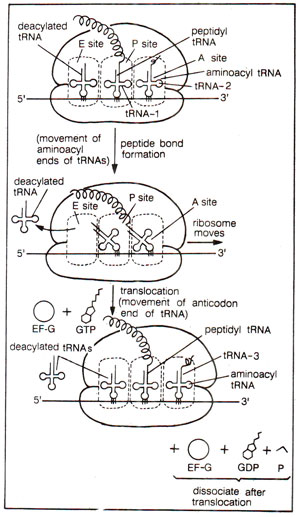
Fig. 34.11. Three site (A, P, E) model for translocation of peptidyl tRNA from A to P site.
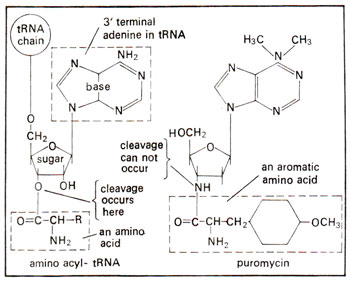
Fig. 34.12. A similarity between amino acyl tRNA and puromycin, so that puromycin can occupy 'A' site, but can not be cleaved due to the presence of N in place of O in RNA.
The peptidyl tRNA present at 'A' site is now translocated to 'P' site. For translocation of peptidyl tRNA from 'A' site to P site, there are two models available : (i) According to two sites (A, P) model, deacylated tRNA is liberated from 'P' site, and with the help of one GTP molecule and an elongation factor EF-G (called EF-G, since it was isolated as GTP requiring factor; also earlier called transfer factor, TFII or translocase), the peptidyl tRNA is translocated from 'A' to 'P' site (Fig. 34.10). Thus according to this model, tRNA is either entirely in the A site or entirely in the P site, (ii) According to newer three sites (A, P, E) model, initially the aminoacyl end of the t-RNA bound to A-site moves to the P site on 50S subunit at the time of peptide transfer (before translocation), but only later during translocation, the anticodon end of this tRNA moves from A to P site on 30S subunit. Only this later step requires action of elongation factor EF-G. In this model thus, there is an intermediate state, when anticodon end of tRNA is still on A site (on 30S subunit), while the aminoacyl end occupies P site (on 50S subunit see Fig. 34.11). A third tRNA binding site, 'E' (Exit) was also recognized, through which tRNA leaves the ribosome. E site is found mainly on 50S subunit, and deacylated tRNA interacts with it through its CCA sequence at its 3' end. The movement of deacylated tRNA from P to E site also occurs in two steps : the aminoacyl end moves to E site during peptide bond formation, and the anticodon end leaves the P site (on 30S) only during translocation. This three site model suggests that tRNAs interact mainly at their ends. It also assumes that a tRNA remains bound to ribosome at one or the other of its two ends (aminoacyl end, anticodon end), and does not leave ribosome at both its ends simultaneously (either on A or P site). The two step transfer of tRNAs "from A to P site and from P to E site could result from reciprocating motions of the two subunits of a ribosome. It means that SOS and 30S subunits move alternately and not simultaneously.
The requirement of EF-G and GTP for translocation was revealed by the use of antibiotic puromycin, which closely resembles AA-tRNA in structure (Fig. 34.12) and can, therefore, occupy 'A' site and form peptidyl puromycin. The later can not form further peptide bond and is released prematurely causing lethality. This puromycin reaction is dependent on the presence of EF-G and GTP, suggesting their significance in translocation process.

Fig. 34.10. Translocation of peptidyl tRNA from 'A' site to 'P' site with the help of elongation factor EF-G and GTP.

Fig. 34.11. Three site (A, P, E) model for translocation of peptidyl tRNA from A to P site.

Fig. 34.12. A similarity between amino acyl tRNA and puromycin, so that puromycin can occupy 'A' site, but can not be cleaved due to the presence of N in place of O in RNA.
The above account of elongation of polypeptide suggests that for the formation of one peptide bond, one ATP molecule (for activation of amino acid) and two GTP molecules (one for transfer of AA-tRNA to 'A' site and the other for translocation of peptidyl tRNA from 'A' to 'P' site) are required. However, in 1993 evidence has been made available, which suggests that the number of GTP molecules for the formation of one peptide bond can be three on even more. For instance, it is suggested that for the transfer of one AA-tRNA, two molecules of che binary complex, EF-Tu-GTP, may be required, so that the total GTP molecules for one peptide bond will be three (two GTP for transfer of AA-tRNA and one GTP for translocation of peptidyl tRNA).





