Gene transfer methods in plants
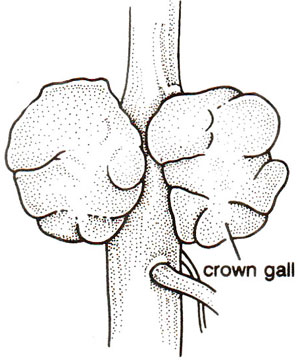
Fig. 42.4. Tumour formation and leafy structure, on the stem of a plant, induced by Agrobacterium tumefaciens.
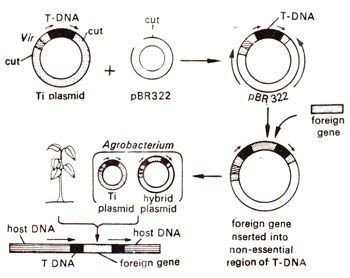
Fig. 42.5. Different steps for the transfer of a foreign gene into a tobacco plant using A. tumefaciens.

Fig. 42.4. Tumour formation and leafy structure, on the stem of a plant, induced by Agrobacterium tumefaciens.

Fig. 42.5. Different steps for the transfer of a foreign gene into a tobacco plant using A. tumefaciens.
Vectors based on Ti and Ri plasmids have been designed with the following properties : (i) they do not cause the disease, since they are disarmed by removing genes causing the disease; (ii) they carry sites for insertion of foreign gene intended to be transferred and (iii) they carry selectable markers (genes which will help in selecting the transformed cells), a powerful promoter like 35S (for high level of expression of marker) derived from cauliflower mosaic virus (CaMV), and a polyadenylation signal (for adding poly A to mRNA). The most commonly used selectable marker is neomycin phosphotransferase (npt II)imparting resistance to kanamycin, so that the transformed cells will grow in a medium supplemented with kanamycin. A vector, so designed, after insertion of the foreign gene, is transferred to A. tumefaciens or A. rhizogenes, as the case may be, and then the bacterium is used for infecting the host cells to which the gene is to be transferred (Fig. 42.5).
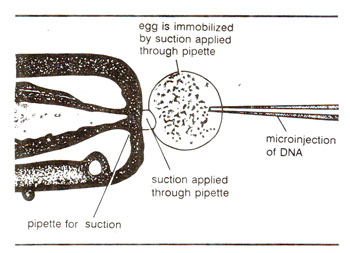
Fig. 42.1. Microinjection of foreign DNA into a fertilized egg for transfer of a gene leading to the production of transgenic animals.
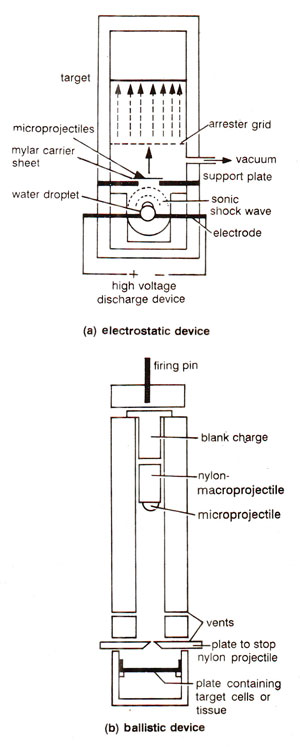
Fig. 42.6. Particle guns for accelerating microprojectiles carrying a gene of interest to the target cells (for transfer of gene).
Transfer of genes using physical delivery methods.
Although Agrobacterium mediated gene transfer was extensively utilized for production of transgenic dicotyledonous plants, it was not successful in monocotyledonous plants, since Agrobacterium does not infect them. Therefore, for cereals and also for some dicots (e.g. legumes), where regeneration was difficult, transgenic plants could not be produced using Agrobacterium. In view of this, DNA mediated gene transfer (DMGT) was successfully tried in a wide range of plants including both monocotyledons and dicotyledons. These DMGT methods include the following :
(i) PEG (polyethylene glycol) stimulated DNA uptake involves the use of 15-25% PEG, which stimulates uptake of DNA by endocytosis without any gross damage to protoplasts. The transformed protoplasts can be selected on a selection medium,
(ii) Microinjection involves injection of DNA, using micropipettes with 0.5-10 μm diameter tip. In this technique, the recipient cells are immobilized on a solid support (cover slip or slide, etc.) or artificially bound or held by a pipette under suction (Fig. 42.1). Specially designed micromanipulators are used for this purpose,
(iii) Macroinjection of DNA, employing needles with diameters greater than cell diameter, has been tried in rye (Secale cereale). DNA was injected into the stem below the immature floral meristem, so as to reach the sporogenous tissue, leading to the production of transgenic rye plants. Unfortunately this technique was not successful when tried with other cereals, making the validity of earlier experiments doubtful,
(iv) Microprojectiles (gold or tungsten particles coated with DNA, 1-3 |im in diameter) are carried by a 'macroprojectile' (or bullet), and are accelerated into target cells, where they penetrate the cell wall, leaving DNA to be incorporated (Fig. 42.6). This technique has been successfully used for transformation in soybean, tobacco, maize, rice, wheat, oats and tall fescue. This method is now believed to be universal in its application and will be extensively utilized in 1990s for gene transfer in plants, irrespective of species and genotype,
(iv) Liposome mediated gene transfer involves encasement of DNA in lipid bags, which can be made to fuse with protoplasts with the help of a peg. This technique has been used successfully for gene transfer in a number of plant species.




