Signal transduction by oncoproteins (G proteins)
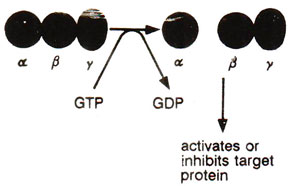
Fig. 45.9. A typical trimeric (α β γ) G protein, where GTP binding releases α subunit from β γ dimer; GTP-α subunit or βγ dimer becomes free to act upon target proteins.
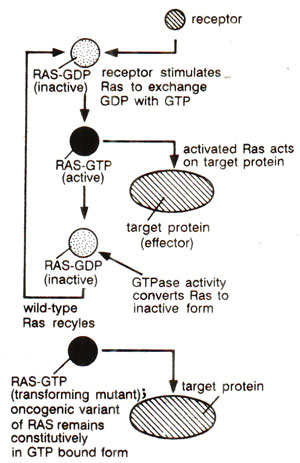
Fig. 45.10. Activation of Ras, which stimulates replacement of of GDP by GTP; the active protein recognizes its effector and GTP is cleaved into GDP, so that resulting inactive Ras is recycled; transforming Ras mutants do not hydrolyze GTP, so that Ras remains permanently in the active form.
G proteins are activated, when GTP binds to the αsubunit (by displacing GDP) and causes its dissociation from the β γ dimer (Fig. 45.9). Such an activation is common to a variety of GTP binding proteins. The separated βγ dimer may carry the message from receptor to effector, although in some cases αsubunit may do this job.

Fig. 45.9. A typical trimeric (α β γ) G protein, where GTP binding releases α subunit from β γ dimer; GTP-α subunit or βγ dimer becomes free to act upon target proteins.

Fig. 45.10. Activation of Ras, which stimulates replacement of of GDP by GTP; the active protein recognizes its effector and GTP is cleaved into GDP, so that resulting inactive Ras is recycled; transforming Ras mutants do not hydrolyze GTP, so that Ras remains permanently in the active form.




