Vitamin A and Vision
While vitamin A, as retinoic acid, has important hormonal
actions (which are not discussed here), its best known
function is in vision. Within photoreceptor cells of the
retina, and even in certain bacteria, vitamin A aldehyde
(retinal, Fig. 1) forms a Schiff base with specific lysine side
chains of the light receptor proteins. Two of the best known
of these receptors are rhodopsin, the pigment present in the
rod cells of the mammalian retina, and bacteriorhodopsin,
the light receptor of the purple membranes of certain
salt-tolerant bacteria. In both of these cases, the protein
consists of a similar bundle of seven connected helical
segments that pass through a membrane. The retinal Schiff
base is inside the bundle, held rigidly in a small “box.” In
both cases, a particular stereoisomer of retinal is present.
In bacteriorhodopsin it is the all-
trans isomer pictured in
Fig. 1, but in rhodopsin it is the 11-
cis isomer shown in
Fig. 19. Upon absorption of light, this isomer is converted
almost instantaneously into the all-
trans form as shown in
Fig. 19. The all-
trans retinal then leaves the photoreceptor
and is replaced with a new molecule of the 11-
cis isomer
before the photoreceptor can act again. In bacteriorhodopsin,
absorption of light converts the all-
trans retinal into the 13-
cis isomer within about three trillionths of
a second. In both cases, the change in shape of the retinal
upon absorption of light induces a small alteration in the
geometry and chemical properties of the photoreceptor
protein that surrounds the light-absorbing molecule. This
is enough to start a chain of signaling events in the retina
that leads to a nerve impulse being sent to the brain. In the
bacteria, the light absorption is used in a different way to
pump a proton from the inside of the cell across the membrane
to the outside. The resulting gradient of hydrogen
ions (positive charges) across the membrane represents
a store of protonic energy similar to that in an electrical
condenser. It is used by these cells as a source of energy.
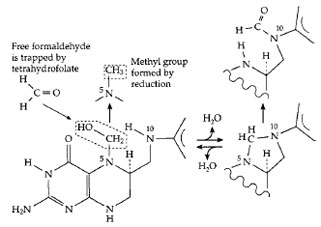 |
| Figure 18 The functioning of tetrahydrofolates (THF) in oxidation
and reduction of single-carbon fragments. A PLP-dependent
enzyme cleaves serine (Fig. 14), releasing formaldehyde, which
combines in the active center with THF. Formic acid can be converted
to formyl-THF. The various THF derivatives supply singlecarbon
fragments for many biosynthetic processes. |
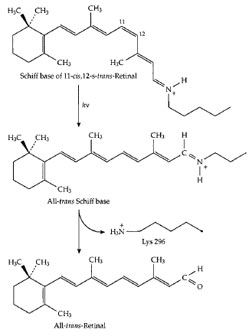 |
| Figure 19 The structural change that takes place in the Schiff
base of retinal (vitamin A aldehyde) that is formed with specific
lysine side chains of the visual pigment proteins upon absorption
of a quantum of light. This change triggers a cycle of alterations
in the protein that initiates an impulse in the optic nerve. |






