What is Light?
Light is an electromagnetic radiation, with wave and particle properties. The electromagnetic radiation has a spectrum or wavelength distribution from short wavelength (10
-6 nm, gamma and x-rays) to long wavelength (10
15 nm, long radio waves). About 99% of the Sun’s radiation is in the wavelength region from 300 to 4000 nm and it is called the broadband or total solar radiation. Within this broadband, different forms of energy exist, which can be associated with specific phenomena such as harmful and potentially mutagen ultraviolet radiation (UV 100–400 nm), sight (visible light 400–700 nm), and heat (infrared radiation 700–4000 nm) (Figure 5.1). Therefore, what we see as visible light is only a tiny fraction of the electromagnetic spectrum; detecting the rest of the spectrum requires an arsenal of scientific instruments ranging from radio receivers to scintillation counters.
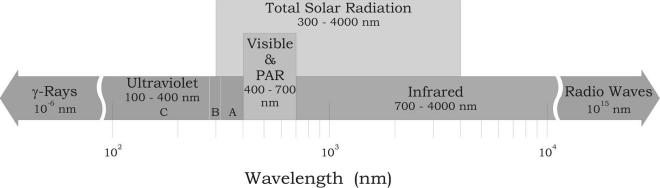
FIGURE 5.1 The electromagnetic spectrum from γ-rays (10-6) to radio waves (1015).
Ultraviolet light is arbitrarily broken down into three bands, according to its anecdotal effects. UV-A (315–400 nm) is the least harmful and the most commonly found type of UV light, because it has the least energy. UV-A light is often called black light, and is used for its relative harmlessness and its ability to cause fluorescent materials to emit visible light, thus appearing to glow in the dark. Most phototherapy and tanning booths use UV-A lamps. UV-B (280–315 nm) is typically the most destructive form of UV light, because it has enough energy to damage biological tissues, yet not quite enough to be completely absorbed by the atmosphere. UV-B is known to cause skin cancer. As most of the extraterrestrial UV-B light is blocked by the atmosphere, a small change in the ozone layer could dramatically increase the danger of skin cancer. Short wavelength UV-C (200–280 nm) is almost completely absorbed in air within a few hundred meters. When UV-C photons collide with oxygen molecules, the O-O bond is broken, and the released O atom reacts with O
2 molecule (and for energetic reasons with a collision partner M) and forms ozone (O
3). UV-C is almost never observed in nature, because it is absorbed very quickly. Germicidal UV-C lamps are often used to purify air and water, because of their ability to kill bacteria.
Infrared light contains the least amount of energy per photon of any other band. Because of this, an infrared photon often lacks the energy required to pass the detection threshold of a quantum detector. Infrared is usually measured using a thermal detector such as a thermopile, which measures temperature change due to absorbed energy. As heat is a form of infrared light, far infrared detectors are sensitive to environmental changes, such as someone moving in the field of view. Night vision equipment takes advantage of this effect, amplifying infrared to distinguish people and machinery that are concealed in the darkness. Little of the ultraviolet radiation (UV-A and UV-B) and infrared are utilized directly in photosynthesis.
Whether transmitted to a radio from the broadcast station, heat radiating from the oven, furnace or fireplace, x-rays of teeth, or the visible and ultraviolet light emanating from the Sun, the various forms of electromagnetic radiation all share fundamental wave-like properties. Every form of electromagnetic radiation, including visible light, oscillates in a periodic fashion with peaks and valleys, and displays a characteristic amplitude, wavelength, and frequency. The standard unit of measure for all electromagnetic radiation is the magnitude of the wavelength (l) and is measured by the distance between one wave crest to the next. Wavelength is usually measured in nanometers (nm) for the visible light portion of the spectrum. Each nanometer represents one-thousandth of a
micrometer. The corresponding frequency (n) of the radiation wave, that is, the number of complete wavelengths that passes a given point per second, is proportional to the reciprocal of the wavelength. Frequency is usually measured in cycles per second or Hertz (Hz). Thus, longer wavelengths correspond to lower frequency radiation and shorter wavelengths correspond to higher frequency radiation. A wave is characterized by a velocity (the speed of light) and phase. If two waves arrive at their crests and troughs at the same time, they are said to be in phase.
An electromagnetic wave, although it carries no mass, does carry energy. The amount of energy carried by a wave is related to the amplitude of the wave (how high is the crest). A high energy wave is characterized by high amplitude; a low energy wave is characterized by low amplitude. The energy transported by a wave is directly proportional to the square of the amplitude of the wave.
The electromagnetic wave does not need any medium for its sustaining; unlike the sound, light can travel in the vacuum.