Introduction to Glycoconjugates and Carbohydrates
The utilization of carbohydrates as food sources, sweetening agents, and clothing materials dates back several thousand years. Likewise, the production of beer and wine was known in ancient times although the relationship to saccharides and fermentation was not appreciated then. Manufacture of sugar as a sweetener including refinements such as decolorization with charcoal also has ancient origins. The commercial importance of carbohydrates extends beyond the food industry to textiles, pharmaceuticals, and a wide range of chemicals.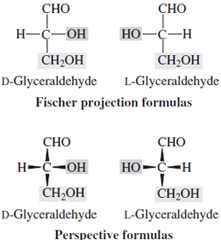 |
| Figure 1 Projection formulas of D- and L- glyceraldehyde. |
An appreciation of the diversity available in such structures began about 1900 with the work of van’t Hoff on the tetrahedral carbon atom, extrapolated by Emil Fischer in his elucidation of the stereochemistry of glucose. During that series of experiments, Fischer was able to deduce the relative positions of all of the hydroxyls at the asymmetric centers and could relate glucose to glyceraldehyde (Fig. 1). However, he was unable to provide an absolute stereo structure for glyceraldehyde and thus had to decide between mirror image structures for natural glucose. That he chose the correct one may reflect little more than that he was right handed. He did synthesize all of the possible aldohexoses.




