Internal Features
Axoneme

FIGURE 2.29 Transmission electron microscopy image of a Dunaliella sp. flagellum in transverse section, showing the homogeneous fuzzy coating of its membrane. (Bar: 0.10 µm)
The movements of the flagella are generated by a single functional unit, the axoneme, which consists
of a long cylinder, from 10 to 100 µm long, with a 0.2 µm diameter. Its structure, as seen in cross-sections by electron microscopy, is almost ubiquitous: it is made of nine equally spaced outer
microtubule doublets (A and B) approximately 40 nm in diameter surrounding two central microtubules,
the central pair (Figure 2.29). This arrangement is maintained by a delicate series of linkages
to give the classical 9+2 pattern. The nine outer doublets are numbered starting from
number 1 located in the plane orthogonal to the plane including the central pair and counting clockwise
when looking from the tip of the flagellum. The former plane allows the definition of the curvature
directions during beating as left or right relative to it. Doublets are transiently linked by
outer/inner Dynein arms (ODA and IDA) that represent the flagellar motor, and permanently interconnected by nexin links; the radial spokes connect the central pair to the peripheral microtubules of the outer doublets.

FIGURE 2.29 Transmission electron microscopy image of a Dunaliella sp. flagellum in transverse section, showing the homogeneous fuzzy coating of its membrane. (Bar: 0.10 µm)
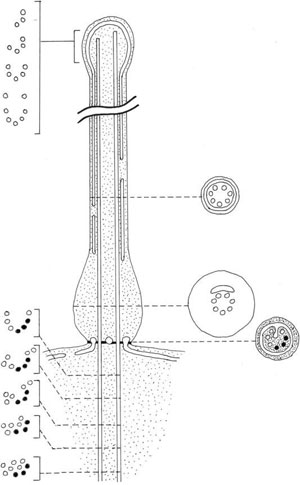
FIGURE 2.36 Schematic drawing of the generalized representation of the structure of the haptonema of Chrysochromulina.
Divergence from the basic 9+2 pattern are rare, but include the spermatozoid
of some centric diatoms (9+0) and the chlorophyta Golenkinia minutissima (9+1), as well as the
haptonema of the Prymnesiophyceae (Figure 2.36). This structure develops between the two flagella
of these algae, and it is sometimes longer than the flagella themselves. It resembles a flagellum,
but contains a central shaft of six to eight microtubules arranged in a cylinder, with no
doublets. In transverse section, the microtubules are disposed in an arc of a circle or in a ring
and are surrounded by a limb of the smooth endoplasmic reticulum. The distal part of the haptonema
is fairly straightforward. It is surrounded by plasma membrane, which is continuous over
the tip of the haptonema and may be smooth, drawn into a tip or form a spathulate projection.

FIGURE 2.36 Schematic drawing of the generalized representation of the structure of the haptonema of Chrysochromulina.
The bulk of axonemal proteins (70%) is made of tubulins, the building blocks (heterodimers)
that polymerize linearly to form microtubules. Those tubulins, which constitute the wall of microtubules belong to the a and b families, whose sequences have been conserved during evolution
(other families, g, d, and e, are responsible for microtubule nucleation at the level of the basal
bodies/centrosomes). A large molecular diversity among tubulins is generated by a series of
post-translational modifications such as acetylation, detyrosylation, polyglutamylation, or polyglycylation. Tektin filaments are present at the junction between the A and B microtubules of
each doublet. The internal and external arms that graft to the peripheral doublets represent
10–15% of the global protein mass of axonemes and are essentially formed by the “dynein-
ATPases” motor (the Greek word “dyne” means force). Microtubular dyneins are large multimolecular complexes with a pseudo-bouquet shape, and a molecular mass ranging from 1.4
(bouquets with two heads) to 1.9 MDa (bouquets with three heads) for the whole molecule, and
approximately 500 kDa for the largest subunits containing the ATP hydrolysis site. The size of
both outer and inner dynein arms is approximately 50 nm. Among the 250 different polypeptides
present in the axoneme, as estimated by bidimensional electrophoresis, only a few have been
associated with a function.








