Male sterility in plants
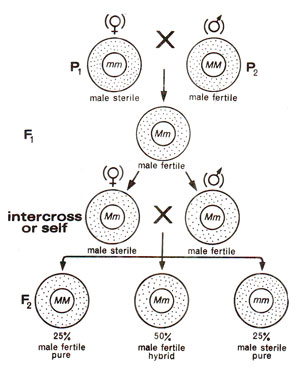
Fig. 18.12. Inheritance of genetic male sterility in plants.
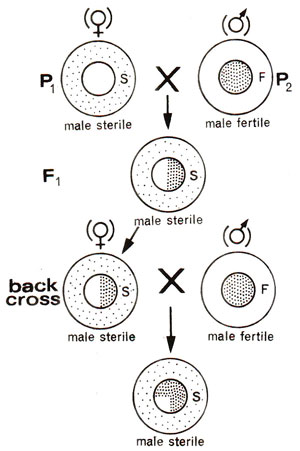
Fig. 18.13. Maternal inheritance of cytoplasmic male sterility in plants.
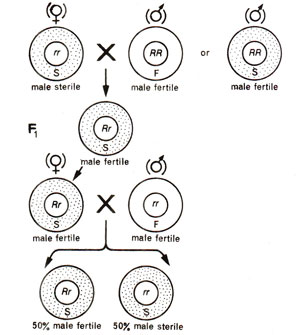
Fig. 18.14. Inheritance of cytoplasmic male sterility and restoration of fertility due to restorer gene.
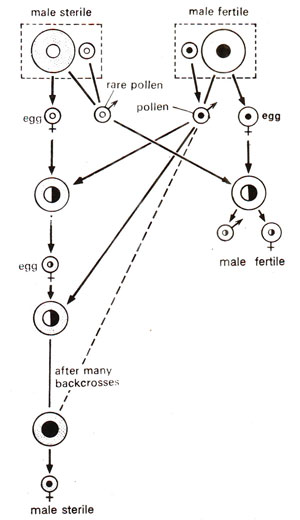
Fig. 18.15. Reciprocal crosses between male sterile and male fertile plants (using rare pollen from male sterile). Figure shows a number of back crosses of male sterile as female parent, substituting the nucleus from male fertile. Note that the substitution of male fertile nucleus does not restore fertility in male sterile.
- Genetic male sterility. In this type, male sterility is controlled by a single gene and is recessive to fertility, so that the F1individuals would be fertile. In the F2 generation, the fertile and sterile individuals will segregate in 3 : 1 ratio (Fig. 18.12).
- Cytoplasmic male sterility. In several crops like maize, cytoplasmic control of male sterility is known. In such cases if female parent is male sterile, F1 progeny would always be male sterile (Fig. 18.13), because cytoplasm is mainly derived from egg obtained from male sterile female parent.
- Cytoplasmic-genetic male sterility. In certain other cases, although male sterility is wholly controlled by cytoplasm, but a restorer gene if present in the nucleus will restore fertility. For instance, if female parent is male sterile, then genotype (nucleus) of male parent will determine the phenotype of F1 progeny. The male sterile female parent will have the recessive genotype (rr) with respect to restorer gene. If male parent is RR, F1 progeny would be fertile (Rr). On the other hand, if male parent is rr, the progeny would be male sterile. If F1 individual (Rr)is testcrossed, 50% fertile and 50% male sterile progeny would be obtained (Fig. 18.14).
During 1990-92, transgenic male sterile and fertility restorer plants have also been produced in Brassica napus, by transfer of 'barnase' and 'barstar' genes from Bacillus amyloliquefaciens (for more details consult Genetic Engineering and Biotechnology 4. Gene Transfer Methods and Transgenic Organisms).
Cytoplasmic male sterility in maize. Rhoades in 1933, reported the analysis of first cytoplasmic male sterile plants in maize and demonstrated that male sterility was contributed by female parent and that nuclear genes had no influence. This was shown by crossing male sterile plants with wide range of fertile males and by observing that in subsequent generations all progenies were male sterile.
In maize, three male sterile sources (cms) are known, which are called T, C and S. The normal male fertile cytoplasm is called N cytoplasm.

Fig. 18.12. Inheritance of genetic male sterility in plants.

Fig. 18.13. Maternal inheritance of cytoplasmic male sterility in plants.

Fig. 18.14. Inheritance of cytoplasmic male sterility and restoration of fertility due to restorer gene.

Fig. 18.15. Reciprocal crosses between male sterile and male fertile plants (using rare pollen from male sterile). Figure shows a number of back crosses of male sterile as female parent, substituting the nucleus from male fertile. Note that the substitution of male fertile nucleus does not restore fertility in male sterile.
(a) Role of mitochondria. Recent studies have proved that factors responsible for cytoplasmic male sterility are located in mitochondrial DNA (mtDNA). Six kinds of evidences proved mitochondrial role.
- When mitochondrial, DNA was studied in three male sterile cytoplasms (T, C and S) and normal male fertile (N) cytoplasm, it was found that mt DNAs differed in four cytoplasms. It is also apparent that mtDNA from a particular cms (cytoplasmic male sterile) source is stable and conserved. For instance Texas (T) cytoplasm, even after 20 generations of backcrosses had same mtDNA from different T sources. However, when chloroplast DNA (ctDNA) was similarly studied, very little, if any differences were found between ct DNA from four different sources (N, T, C, S). Such an observation was used as an evidence to suggest that cytoplasmic male sterility in higher plants may be associated with mt DNA.
- Association of mtDNA has also been shown with disease susceptibility, which itself is associated with cytoplasmic male sterility (cms). Both pathogens, one causing leaf blight disease and the other causing yellow leaf blight are known to produce host specific toxins. These toxins affect the mitochondria of T cytoplasm, but not of N cytoplasm. Such an effect of toxins on mitochondria was observed both inside the cell (in vivo systems) and in cell free mitochondrial preparations (in vitro systems).
- The reaction of toxins on mitochondria was modified in cytoplasmic male steriles, which were restored to fertility with the help of restorer genes (Rf1, Rf2-nuclear genes; if these genes are present, male sterile cytoplasm does not express itself). This suggests that restorer genes help restore fertility through' their action on mitochondria.
- When T-cytoplasm cells in culture were treated with toxin and resistant cells were selected, then mitochondria of these resistant cells were unaffected by toxin. Mature plants derived from these resistant cells selected originally from male sterile plants, were fertile and disease resistant. It could be shown that this change involved changes in mitochondria only.
- It has been shown in several organisms that there are several proteins synthesized under the control of mtDNA and these proteins include cytochrome oxidase, cytochrome b and ATPase. There are evidences available that in T cytoplasm, cytochrome oxidase, cytochrome b and ATPase get affected. Cytoplasm specific proteins characteristic of individual cytoplasms have been shown to be present in N, T, C and S cytoplasms, although the sites of action or function of these distinguishing proteins are not known.
- When microsporogenesis was studied in anthers of T and N cytoplasms, mitochondrial degeneration was observed in tapetum and middle layers of anther at the tetrad stage in T cytoplasm and not in N cytoplasm. This degeneration includes, swelling of mitochondria, loss of cristae and internal disorganization. Contrary to this, plastids and other organelles were not affected.




