Colloids
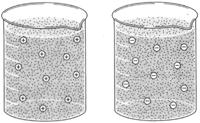 |
| Figure 8-1 Because like charges repel, dispersed (cotloidal) particles having the same electrical charge tend to remain dispersed |
Some of the substances of protoplasm are in solution, some in the form of ions, and some are colloids. Colloids are either aggregates of molecules or very large molecules( such as proteins) dispersed in water. Colloidal particles, while not in true solution, remain dispersed for a long time. Each colloid has an electrical charge, either positive or negative. The fact that like charges repel helps maintain the colloidal dispersion.
The finely divided state of colloidal particles is significant to the events that occur in protoplasm because this state offers a large surface area for substance interaction. Consider a cube 1 centimeter in length on each side. Such a cube has 6 square centimeters of surface area. If the cube were cut in two, 8 square centimeters of surface area would be exposed. If cut through again, 10 square centimeters of surface area would be exposed (see figure 8-2). Without increasing the amount of substance, the surface area of the substance is increased by dividing the substance into smaller parts. If this same 1 cubic centimeter of material were divided into the colloidal state, 60,000 square centimeters of surface area would be exposed.
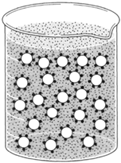 |
| Figure 8-3 Particles in colloidal dispersion can be keipnt a dispersed state by surrounding each particle with an emulsifying agent such as soap |
A colloid is constituted of two parts called phases: a continuous phase and a dispersed phase. Oil can be dispersed in water by agitating the water: but when the agitation ceases,t he droplets of oil promptly coalesceI.f a third substance called an emulsifying agent is added to the mixture, however, the oil will disperse and remain so in a colloidal state. The emulsifying agent surrounds each minute droplet of oil and prevents the droplets from coming together again. In figure 8-3, oil is the dispersed phase and water the continuous phase. This could be reversed, however, by having water dispersed in oil.
A watery colloid is called a sol; a viscous colloid, a gel. Protoplasm is a mixture of sols and gels, and one may change into the other and back numerous times.




