The importance of soil pH
Content
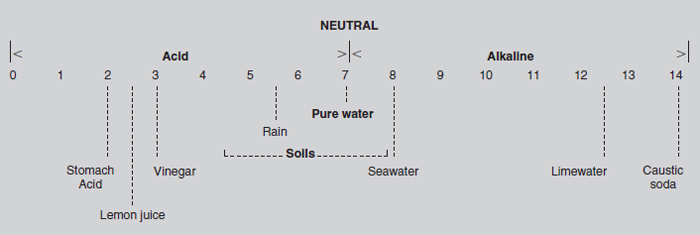
The pH scale is a means of expressing the degree of acidity or alkalinity of the soil. Temperate area soils are usually between pH 4 and 8; the vast majority are between 5.5 and 7.5. The ideal growing condition for most plants is a soil of about pH 6.5, which is slightly acid. At this level most of the plant nutrients are available for uptake by the roots. Alkaline conditions are usually created by the presence of large quantities of calcium (‘lime’), which interferes with the uptake and utilization of several plant nutrients. Acidity, alkalinity and the pH scale Pure water is neutral, i.e. neither acid nor alkaline. It is made up of two hydrogen atoms and one oxygen atom, expressed as the familiar formula H2O. Most of the water is made up of water molecules in which the atoms stay together, but a tiny proportion dissociates, i.e. they form ions. Equal numbers of positive ions, cations, and negative ions, anions, are formed. In the case of water, an equal number of hydrogen cations, H+, and hydroxyl anions, OH-, exist within the clusters of water molecules (H2O). When, as in water, the concentration of hydrogen ions is the same as that of hydroxyl ions, there is neutrality. Many compounds form ions as they dissolve and mix with water to produce a solution. If the concentration of hydrogen ions is increased, the concentration of hydroxyl ions decreases and acidity increases. Likewise, the addition of hydroxyl ions and a decrease in hydrogen ions leads to increased alkalinity. All acids release hydrogen ions when dissolved in water. Whereas a strong acid (such as hydrochloric acid) fully dissociates when dissolved (i.e. every molecule splits into ions), only a part of a weak acid (such as carbonic or acetic acid) breaks up into ions. Bases, such as caustic soda, dissolve in water and increase the concentration of hydroxyl ions. The pH scale expresses the amount of acidity or alkalinity in terms of hydrogen ion concentration. pH values less than 7 are acid and the lower the figure the greater the acidity. Values greater than 7 indicate increasing alkalinity and pH 7 is neutral. In order to present the scale simply, negative logarithms are used: ‘pH’ is the negative logarithm, the mathematical symbol for which is ‘p’, of the hydrogen ion concentration, abbreviated as ‘H’. It is important to note that, as the scale is logarithmic, a one-unit change represents a ten-fold increase or decrease in hydrogen ion concentration and a change of two units represents a hundred-fold change. Thus a solution of pH 3 is ten times more acid than one of pH 4, one hundred times more acid than pH 5, and a thousand times more than pH 6.
|
|||||||||||||||||||||||||