Algae and the Oxygen/Carbon Cycles
The oxygen and carbon cycles are closely related, because they are directly associated with photosynthesis and respiration processes.
The natural oxygen cycle is determined by the aerobic respiration of glucose (taking place in all living organisms), which consumes oxygen in free form (O
2) using it as electron sink and produces carbon dioxide and water, and by photosynthesis, which consumes carbon dioxide and water to produce carbohydrates and molecular oxygen as a by-product.
Today oxygen constitutes about 21% of the atmosphere, 85.8% of the ocean, and 46.7% by volume of the Earth’s crust. It has not always been like that. The primordial atmosphere of the Earth is thought to have contained mainly CO
2, N
2, H
2O, and CO with traces of H
2, HCN, H
2S, and NH
3, but to have been devoid of O
2 (only small amounts were derived from the photolysis of water), thus being neutral to mildly reducing.
Today, the atmosphere contains 78% N
2, 21% O
2, and 0.036% CO
2 by volume, and is strongly oxidizing. All of the molecular oxygen present in the Earth’s atmosphere has been produced as the result of oxygenic photosynthesis, the source of the original O
2 being photosynthetic activity in the primordial oceans.
The development of aquatic photosynthesis coincided with a long and reasonably steady drawdown of atmospheric CO
2, from concentration approximately 100-fold higher than in the present-day atmosphere to approximately half of the present levels. This drawdown was accompanied by a simultaneous evolution of oxygen from nil to approximately 21%, comparable to that of the present day. The current atmospheric oxygen concentration is maintained in equilibrium between the production by photosynthesis and the consumption by respiration, with annual fluctuations of ±0.002%. Over geological time scales, the drawdown of CO
2 was not stoichiometrically proportional to the accumulation of O
2 because photosynthesis and respiration are but two of the many biological and chemical processes that affect the atmospheric concentrations of these two gases. The removal rate of CO
2 from the atmosphere by photosynthesis on land is about 60 gigatons C yr
-1, worldwide.
The concentration of oxygen in the oceans (85.8%) is influenced horizontally and vertically by physical features such as the thermocline (i.e., a layer in a large body of water, such as a lake, that sharply separates regions differing in temperature), which isolates deep water from exchange
with the atmosphere and can be a zone of significant decomposition causing an oxygen minimum. Oxygen is only sparingly soluble in water (oxygen solubility is inversely proportional to the temperature) and diffuses about 104 times more slowly in water than air. Deep water masses are produced at the sea surface in the polar zones where cooling gives rise to increased gas solubility and convection currents. These waters remain largely intact and move through the ocean basins with their oxygen concentration decreasing with time due to the decomposition of organic matter.
Carbon, the key element of all life on Earth, has a complex global cycle that involves both physical and biological processes, made up of carbon flows passing back and forth among four main natural reservoirs of stored carbon: the atmosphere, storing 735 gigatons (0.001%) of the world’s carbon as carbon dioxide (CO
2), carbon monoxide (CO), methane (CH
4), longer chain volatile hydrocarbons, and halogen compounds (CFC and HCFC compounds); living organisms, storing 8000 gigatons (0.001%) of the world’s carbon as compounds like fats, carbohydrates, and proteins; the hydrosphere, storing 39,000 gigatons (0.06%) of the world’s carbon, as dissolved carbon dioxide; the lithosphere, storing 1000 gigatons (0.002%) of the world’s carbon in the form of fossils (e.g., oil, natural gas, lignite, and coal), and 62,000,000 gigatons (99.9%) in sedimentary rocks (e.g., limestone and dolomite). Carbon is also present in the mineral soil, in the bottom sediments of water bodies, in peat, bogs and mires, in the litter and humus, which contain 3000 gigatons (0.005%) of the world’s carbon.
Carbon dioxide enters the ocean from the atmosphere because it is highly soluble in water; in the sea, free dissolved CO
2 combines with water and ionizes to form bicarbonate and carbonate ions, according to the following equilibrium:
These ions are bound forms of carbon dioxide, and they (especially bicarbonate) represent by far the greatest proportion of dissolved carbon dioxide in seawater. On average, there are about 45 ml of total CO
2 in 1L of seawater, but because of the equilibrium of chemical reactions, nearly all of this occurs as bound bicarbonate and carbonate ions which thus act as a reservoir of free CO
2. The amount of dissolved CO
2 occurring as gas in 1L of seawater is about 0.23 ml. When free CO
2 is removed by photosynthesis, the reaction shifts to the left and the bound ionic forms release more free CO
2; so even when there is a lot of photosynthesis, carbon dioxide is never a limiting factor to plant production. Conversely, when CO
2 is released by the respiration of algae, plants, bacteria, and animals, more bicarbonate and carbonate ions are produced.
According to the general chemical reactions presented earlier, the pH of seawater is largely regulated by the concentrations of bicarbonate and carbonate, and the pH is usually 8±0.5. The seawater acts as a buffered solution, because when CO
2 is added to seawater due to mineralization processes and respiration, the number of hydrogen ions increases and the pH goes down (the
solution becomes more acidic). If CO
2 is removed from water by photosynthesis, the reverse happens and the pH is elevated.
Some marine organisms combine calcium with carbonate ions in the process of calcification to manufacture calcareous skeletal material. The calcium carbonate (CaCO3) may either be in the form of calcite or aragonite, the latter being a more soluble form. After death, this skeletal material sinks and is either dissolved, in which case CO
2 is again released into the water, or it becomes buried in sediments, in which case the bound CO
2 is removed from the carbon cycle. The amount of CO
2 taken up in the carbonate skeletons of marine organisms has been, over geological time, the largest mechanism for absorbing CO
2. At present, it is estimated that about 50 x 10
15 tons of CO
2 occurs as limestone, 12 x 10
15 tons in organic sediments, and 38 x 10
12 tons as dissolved inorganic carbonate.
Calcification is not confined to a specific phylogenetically distinct group of organisms, but evolved (apparently independently) several times in marine organisms. Carbonate sediments blanket much of the Atlantic Basin, and are formed from the shells of both coccolithophorids and foraminifera. As the crystal structures of the carbonates in both groups is calcite (as opposed to the more diagenically susceptible aragonite), the preservation of these minerals and their co-precipitating trace elements provides an invaluable record of ocean history. Although on geological time scales, huge amounts of carbon are stored in the lithosphere as carbonates, on ecological time scales, carbonate formation depletes the oceans of Ca
2+, and in so doing, potentiates the efflux of CO
2 from the oceans to the atmosphere. This calcification process can be summarized by the following reaction:
Among the marine organisms responsible for calcification, coccolithophores play a major role, especially Emiliania huxleyi. When the blooms of this Haptophyta appear over large expanses of the ocean (white water phenomenon), myriad effects on the water and on the atmosphere above can be observed. Although each cell is invisibly small, there can be as many as a thousand billion billion (10
21) of them in a large bloom, and the population as a whole has an enormous impact.
E. huxley blooms are processed through the food web, with viruses, bacteria, and zooplankton all contributing to the demise and decomposition of blooms. Some debris from the bloom survive to sink to the ocean floor, taking chemicals out of the water column. While they live and when they die, the phytoplankton cells leak chemicals into the water.
A bloom can be thought of as a massive chemical factory, extracting dissolved carbon dioxide, nitrate, phosphate, etc. from the water, and at the same time injecting other chemicals such as oxygen, ammonia, DMS, and other dissolved organic compounds into the water. At the same time, the chemical factory pumps large volumes of organic matter and calcium carbonate into the deep ocean and to the ocean floor. Some of this calcium carbonate eventually ends up as chalk or limestone marine sedimentary rocks, perhaps to cycle through the Earth’s crust and to reappear millions of years later as mountains, hills, and cliffs. Coccolithophorids are primarily found at low abundance in tropical and subtropical seas, and at higher
concentrations at high latitudes in midsummer, following diatom blooms. Hence, export of inorganic carbon by diatoms in spring at high latitudes can be offset by an efflux of carbon to the atmosphere with the formation of coccolithophore blooms later in the year.
The contemporary ocean export of organic carbon to the interior is often associated with diatom blooms. This group has only risen to prominence over the past 40 million years.
Coccolithophorid abundance generally increases through the Mesozoic, and undergoes a culling at the Kretaceous/Tertiari (K/T) boundary, followed by numerous alterations in the Cenozoic. The changes in the coccolithophorid abundances appear to trace eustatic sea level variations, suggesting that transgressions lead to higher calcium carbonate fluxes. In contrast, diatom sedimentation increases with regressions and because of the K/T impact, diatoms have generally replaced coccolithophorids as ecologically important eukaryotic phytoplankton. On much finer time scales, during the Pleistocene, it would appear that interglacial periods favor coccolithophorids abundance, whereas glacial periods favor diatoms. The factors that lead to glacial-interglacial variations between these two functional groups are relevant to elucidating their distributions in the contemporary ecological setting of the ocean.
Coccolithophores influence regional and global temperature, because they can affect ocean albedo and ocean heat retention, and have a greenhouse effect. Coccoliths do not absorb photons, but they are still optically important because they act like tiny reflecting surfaces, diffusely reflecting the photons.
A typical coccolith bloom (containing 100 mg m
-3 of calcite carbon) can increase the ocean albedo from 7.5 to 9.7%. If each bloom is assumed to persist for about a month, then an annual coverage of 1.4 x 10
6 km
2 will increase the global annual average planetary albedo by
where 510 x 10
6 km
2 is the surface area of the Earth.
This is a lower bound on the total impact, because sub-bloom concentration coccolith light scattering will have an impact, over much larger areas (estimated maximum albedo impact = 0.21%). A 0.001% albedo change corresponds to a 0.002 W m
-2 reduction in incoming solar energy, whereas an albedo change of 0.21% causes a reduction of 0.35 W m
-2. These two numbers can be compared to the forcing due to anthropogenic addition of CO
2 since the 1700s, estimated to be about 2.5 W m
-2. Coccolith light scattering is therefore a factor of only secondary importance in the radiative budget of the Earth. However, the scattering caused by coccoliths causes more heat and light than usual to be pushed back into the atmosphere; it causes more of the remaining heat to be trapped near to the ocean surface, and only allows a much smaller fraction of the total heat to penetrate deeper in the water. Because it is the near-surface water that exchanges heat with the atmosphere, all three of the effects just described conspire to mean that coccolithophore blooms may tend to make the overall water column dramatically cooler over an extended period, even though this may initially be masked by a warming of the surface skin of the ocean (the top few meters).
All phytoplankton growth removes CO
2 into organic matter and reduces atmospheric CO
2 (by means of photosynthesis). However, coccolithophores are unique in that they also take up bicarbonate, with which to form the calcium carbonate of their coccoliths (calcification process). The coccolithophorid blooms are responsible for up to 80% of surface ocean calcification. In the equilibrium of calcification process, an increase in CO
2 concentration leads to calcium carbonate dissolution, whereas a decrease in CO
2 levels achieves the reverse. While photosynthetic carbon fixation decreases the partial pressure of CO
2 as dissolved inorganic carbon is being utilized, conditions favoring surface calcification by coccolithophorid blooms contribute to the increase of dissolved CO
2.
The relative abundance of the components of the carbonate system (CO
2, H
2CO
3, HCO
-3 ,and CO
2-3) depends on pH, dissolved inorganic carbon, and the total alkalinity, and the equilibrium
between the components can shift very easily from being in one of these dissolved forms to being in another. How much of the total carbon in each form is determined mainly by the alkalinity and by the water temperature? When the seawater carbon system is perturbed by coccolithophore cells removing HCO
-3 to form coccoliths, this causes a rearrangement of how much carbon is in each dissolved form, and this rearrangement takes place more or less instantaneously. The removal of two molecules of HCO
-3 and the addition of one molecule of CO
2 change the alkalinity and this indirectly causes more of the dissolved carbon to be pushed into the CO
2 form. Although the total dissolved carbon is obviously reduced by the removal of dissolved carbon (bicarbonate ions) into solid calcium carbonate, yet the total effect, paradoxically, is to produce more dissolved CO
2 in the water.
In this way, coccolithophore blooms tend to exacerbate global warming by causing increased atmospheric CO
2 (greenhouse effect), rather than to ameliorate it, as is the case when dissolved CO
2 goes into new organic biomass. However, recent work is showing that additional properties of coccoliths may make the situation yet more complicated. Coccolith calcite is rather dense (2.7 kg L
-1 compared to seawater density of 1.024 kg L
-1), and the presence of coccoliths in zooplankton faecal pellets and marine snow (the two main forms in which biogenic matter sinks to the deep ocean) causes them to sink more rapidly. Slow-sinking organic matter may also adhere to the surfaces of coccoliths, hitching a fast ride out of the surface waters. If organic matter sinks faster then there is less time for it to be attacked by bacteria and
so more of the locked-in carbon will be able to escape from the surface waters, depleting the surface CO
2. Probably this co-transport of organic matter with coccoliths offsets the atmospheric CO
2 increase that would otherwise be caused, and makes coccolithophore blooms act to oppose global warming, rather than to intensify it.
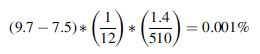 (4.9)
(4.9)



