Polarographic Assays of Mitochondrial Functions
Mitochondrial metabolic functions are primarily concerned with the conservation of energy liberated by the aerobic oxidation of respiring substrates. Energy is conserved in the form that can subsequently be used to drive various cell functions. In recent years, it has been found that mitochondria also play critical roles in various cellular functions, including electrolyte balance, signal transduction, calcium homeostasis, oxidative stress, immunologic defense, and natural aging and/or apoptosis (cf. Lee, 1994). Application of the oxygen electrode technique to study mitochondrial respiration and oxidative phosphorylation was first introduced by Chance and Williams in 1955. The kinetic dependence of mitochondrial respiration on the availability of inorganic phosphate and ADP had been earlier reported by Lardy and Wellman (1952). Together, these studies provided the basis of the concept of respiratory control. The polarographic technique for measuring rapid changes in the rate of oxygen utilization by cellular and subcellular systems is now widely used by many laboratories because of its simplicity and ease of performance. Concurrent monitoring of the metabolic changes induced kinetically by various substrates and reagent(s) can provide invaluable information. This article describes a typical protocol employed in the authors' laboratory for the measurement of respiratory rates and its accompanied oxidative phosphorylation, which are catalyzed by isolated mitochondria. As an example, isolated intact mitochondria were chosen for presentation and discussion. The technique for preparation of isolated intact mitochondria has been described previously (Lee et al., 1993a, b).
The polarographic technique for measuring mitochondrial oxidative changes requires the following four basic components: an oxygen electrode, a closed reaction vessel, a constant voltage source, and a recorder. The Clark-type oxygen electrode is used most commonly (Yellow Springs Instrument Co., Yellow Springs, Ohio, 45387) and consists of a platinum cathode and a Ag/AgCl anode, which is bathed in a half-saturated KCl solution. The tip of the electrode is covered by a polyethylene membrane, which is held firmly over the end of the electrode by a rubber "O" ring. The reaction vessel can be constructed of glass, Plexiglas, or polycarbonate, and the design and the size of the vessel vary, depending on the requirements of the system under investigation. A number of vessels are available commercially. The reaction vessel used routinely in the authors' laboratory was made of polycarbonate and is composed of two parts: a cylindrical open top reaction chamber and a plug that fits in the top of the chamber. The temperature of the reaction chamber is constantly maintained with a water jacket. Constant stirring of the contents of the reaction chamber is accomplished by a disk-shaped, plasticencased magnet located at the bottom of the reaction chamber. The polycarbonate plug, which is secured with a screw, fits tightly in the top of the reaction chamber. The plug has two vertical openings: the larger is fitted with a screw that holds the Clark electrode securely and the smaller one is used for the delivery of substrate(s) and reagent(s) into the reaction mixture. The physical arrangement of the reaction chamber and the oxygen electrode in the assembled form are shown in Fig. 1A. The disassembled form, which shows the details of each individual component, is shown in Fig. 1B.
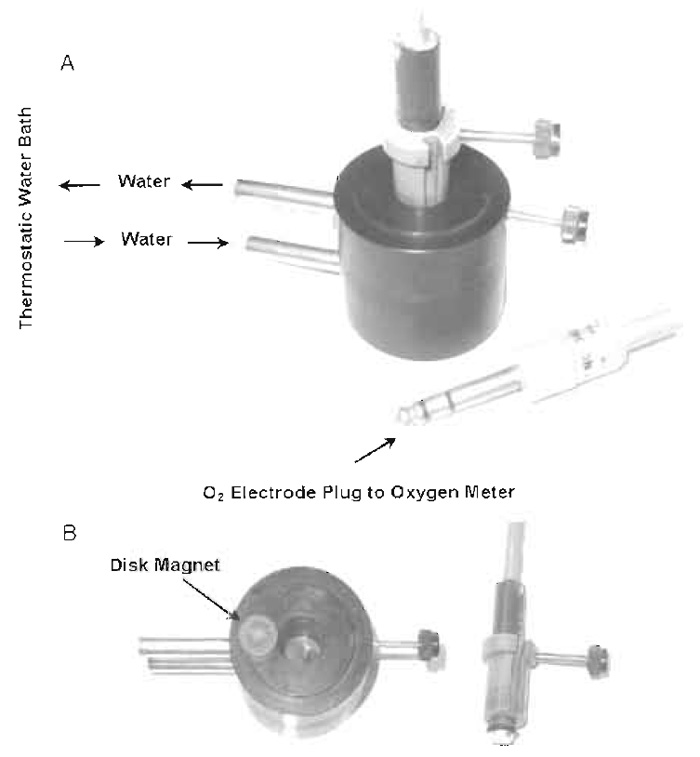 |
| FIGURE 1 Reaction vessel for measurement of oxygen utilization with the oxygen electrode. Oxygen electrode and the reaction chamber are displayed in assembled form (A) and in disassembled form (B). |
At the conclusion of each experiment the reaction chamber is cleaned by first disassembling the plug from the reaction chamber, aspirating the reaction mixture, and washing the plug, oxygen electrode, and the interior of the reaction chamber thoroughly with water. When a water-insoluble reagent is used, initial cleaning is accomplished with ethanol and is then followed with water rinsing. It is essential that no traces of ethanol are left behind as adverse effects may be induced. The reaction vessel is then reassembled and is ready for the next experiment.
Reagents
From Sigma Chemicals Co.: Sucrose (Cat. No. S- 978), bovine serum albumin (BSA, Cat. No. A-4378), EGTA (Cat. No. E-4378), EDTA (Cat. No. ED2SS), adenosine 5'-diphosphate (ADP, Cat. No. A-6646), r-malic acid (Cat. No. M-1000), pyruvic acid (Cat. No. P-2256), β-NADH (Cat. No. 340-110), HEPES (Cat. No. H-3375), carbonyl cyanide 4-trifluoromethoxyphenylhyddrazone (FCCP, Cat. No. C22920), antimycin A (Cat. No. A 8674), oligomycin (Cat. No. O-4876), Tris (Cat. No. T-1503), crystalline Bacillus subtilis protease (Nagarse) from Teikoku Chemical Company, Osaka, Japan, or Sigma P-4789, protease type XXVII (7.6 units/mg solid), phospho(enol)pyruvic acid (PEP, Cat. No. P-0564), pyruvate kinase (Cat. No. P-9136), β-hydroxybutyric acid (Cat. No. H 6501), and lactic dehydrogenase (LDH, Cat. No. L-2375).
III. PROCEDURES
A. Preparation of Solutions
- 0.25M sucrose: Dissolve 171.15 g of sucrose in 1000ml distilled water, filter through a layer of glass wool, add distilled water to 2 liters in a volumetric flask, mix well, and store at 4°C.
- 0.5M Tris-HCl, pH 7.4: Dissolve 30.28g Tris into 400ml distilled water, adjust pH to 7.4 with 4N HCl, add distilled water to 500ml in a volumetric flask, mix well, adjust the pH if necessary, and store at 4°C.
- 0.5M MgCI2: Dissolve 10.17g of MgCl2·6H2O in 100 ml distilled water.
- 0.1M phosphate buffer, pH 7.4: To 800ml distilled water, dissolve 11.83g of Na2HPO4 and 2.245g of KH2PO4 with the aid of magnetic stirrer. Check the pH and adjust with 0.1M HCl or 0.1M NaOH if necessary. Add distilled water to 1000ml in a volumetric flask, mix well, and store at 4°C.
- 0.2M EDTA, pH 7.4: Dissolve 3.72g EDTA in 35 ml of 1 N NaOH. Stir until it dissolves completely; adjust pH to 7.4 with 1 N HCl. Add distilled water to 50ml. Divide into 10 x 5.0ml and store at -20°C.
- 0.1M HEPES, pH 7.4: Dissolve 23.83 g HEPES in 800ml distilled water. Adjust with 1M NaOH to pH 7.4. Add distilled water to 1000ml in a volumetric flask. Mix well and store at 4°C.
- Sucrose/Tris-Cl (S/T) reaction medium: 60ml 0.25M sucrose, 5 ml 0.5M Tris-HCl (pH 7.4), and 35ml distilled water to a final volume of 100ml. Make the S/T medium fresh every day.
- 1.0M pyruvate, pH 7.4: Dissolve 5.5g pyruvic acid in 20 ml of 1 N NaOH; adjust with 5 N NaOH to pH 7.4. Add distilled water to 50ml in a volumetric flask. Mix well, transfer into 10 test tubes with each containing 5 ml, and store at -20°C.
- 0.5M malate, pH 7.4: Dissolve 3.35g of DL-malic acid in 20ml of 1 N NaOH; adjust with 5 N NaOH to pH 7.4. Add distilled water to 50ml in a volumetric flask. Mix well and transfer into 10 test tubes and store at -20°C.
- 0.1M ADP, pH 6.8-7.4: Dissolve 0.48g ADP in 5ml distilled water, adjust with 1N NaOH to pH to 6.8-7.4, and add distilled water to 10ml in a volumetric flask. Mix well, transfer into five test tubes (≈ 2ml/tube), and store at -20°C. The concentration of ADP will be determined spectrophotometrically with the pyruvate kinase coupled with lactate dehydrogenase system (see later).
- 0.1M phosphoenolpyruvate (PEP), pH. 7.4: Dissolve 20.8 mg PEP into 0.6 ml 1N KOH, adjust pH to 7.4 with 5N KOH, and add distilled water to 1.0ml. Mix well, transfer into five vials (0.2 ml/vial), and store at -20°C.
- 1.0mg/ml oligomycin: Dissolve 10mg oligomycin in 10ml absolute ethanol. Store at -20°C.
- FCCP 1 mM: Dissolve 5.03mg FCCP into 20ml absolute ethanol in a glass tube (either dark glass or covered with aluminum foil to protect against light) and store at -20°C.
This assay is based on a pair of coupled reactions. ADP in the presence of an excess amount of (PEP) and pyruvate kinase will be completely converted into ATP and an equal concentration of pyruvate. The pyruvate will then be converted to lactate in the presence of lactate dehydrogenase and an excess amount of NADH. The NAD+ formed is equal to the concentration of lactate. The stoichiometry of ADP to NADH bears a 1:1 relationship. The concentration of ADP is therefore equal to that of NADH oxidized. See the following reaction mixture: 2.8ml S/T medium, 50µl pyruvate kinase (3IU), 10µl lactic dehydrogenase (1IU), 15µl NADH (30mM), 20µl PEP (0.1M), 80µl MgCl2 (0.25 M), and 20 µl KCl (1.0 M). The total volume equals 3.0 ml.
Calibrate the spectrophotometer and the settings of the recorder according to the instruction manuals of the instrument and set the wavelength at 340nm.
- Place the sample cuvette in the sample chamber with 150 µM NADH (15 µl, 30 mM) present; an absorbance in the vicinity of 0.9 is indicated on the instrument and the recorder.
- Turn on the recorder, let it proceed to a constant reading (a minute or two), add 3µl of 0.1M ADP, and stir the solution well with a glass or plastic stirrer; the absorbance at 340nm will decline quickly (when sufficient pyruvate kinase and lactate dehydrogenase are present) followed by a sharp transition to a constant level where there are no more changes in 340nm absorbance. The decline of 340nm absorbance reflects the oxidation of NADH. The extent of absorbance change (i.e., ΔA = 0.63) is dependent on the amount of ADP added into the cuvette.
- The concentration of ADP can therefore be calculated from the absorbance changes at 340nm. The millimolar absorbance coefficient for NADH is 6.22mM -1 cm -1. The ADP concentration = [0.63 / 6.22] x [3.0 / 0.003] = 101 mM.
A simple and rapid method of determining the oxygen content of the reaction medium accurately is by using submitochondrial particles (SMP) with a limiting amount of NADH. The high affinity of SMP for NADH permits a stoichiometric titration of oxygen content. NADH concentration can be determined accurately spectrophotometrically (as shown in the previous section). When limiting concentrations of NADH are added, the change in current, which occurs with complete oxidation of the NADH, can be determined directly. A direct calibration can therefore be obtained. For example, add 0.98ml of air-saturated S/T medium first followed by 10 µl SMP (0.2 mg protein) to the reaction chamber. Allow the reaction mixture to thermo equilibrate; no air bubbles should be trapped in the reaction vessel. Add 5µl NADH (150 µM) to the chamber. Immediately initiated oxygen uptake with a linear kinetics, followed by a sharp transition to a straight line (i.e., oxygen uptake ceases) when all the added NADH is oxidized. This assay can also be used to estimate of back diffusion of oxygen into the reaction mixture. For instance, if there is a back diffusion of oxygen, an increase in oxygen concentration in the reaction mixture will be noted on the left deflection (a negative slop) of the recorder tracing.
Figure 2A shows a recording tracing from a typical experiment utilizing the oxygen electrode apparatus of the type shown in Fig. 1 to determine the respiratory control index (RCI) and P/O ratio of a tightly coupled mitochondrial preparation. Suspend freshly prepared mouse skeletal muscle mitochondria (30-50µl) in an air-saturated Pi-containing isotonic sucrose medium (0.9ml); the addition of substrates [5µl pyruvate (1M) + 5µl malate (0.5M)] causes a slow rate of oxygen uptake (state 4). Subsequent addition of 3µl ADP (100mM) increases the rate (state 3) by ll-fold. Upon the expenditure of the added ADE as indicated by the sharp transition in the polarographic tracing, the rate of oxygen uptake declines to that observed before the addition of ADR The duration of the increased rate of oxygen uptake is proportional to the amount of ADP added to the reaction mixture. The concentration of oxygen consumed is proportional to the amount of ADP phosphorylated to ATR These cycles of stimulation of respiration by the addition of ADP could be repeated several times in a single experiment until all the oxygen in the reaction mixture is consumed (Fig. 2A). The ADP/O [P/O] ratio and RCI can be calculated directly from the oxygen electrode tracing. The dependence of substrate oxidation on the presence of ADP indicates that electron transfer of the respiratory chain and ATP synthesis are coupled to each other. Unless the energy generated during electron transfer is utilized for ATP synthesis or other energy-requiring processes, the oxidation of respiring substrate is restrained.
The respiratory control index is defined as the ratio of respiratory rate in the presence of added ADP (state 3) to the rate either before ADP addition or the rate following ADP expenditure (state 4). State 3 and state 4 respiratory rates are 79 and 7mm/min, respectively. Therefore, the RCI is 79/7 = 11.4. For tightly coupled intact mitochondria as shown in Fig. 2A, the respiratory rates before the addition of ADP and then following the expenditure of ADP are virtually identical. However, if mitochondria are loosely coupled and/or contaminated with ATPase, the respiratory rate following the expenditure of ADP is considerable faster than that with substrate alone before the addition of ADP. The quality of the mitochondrial preparation is readily reflected from the polarographic tracing.
As shown in Fig. 2B, The ADP-induced state 3 respiratory rate declined to the level comparable to state 4 upon the addition of oligomycin. The oligomycininduced inhibition was released by the addition of FCCP. The FCCP-induced rate (usually referred to as state 3U) is virtually identical to that at state 3.
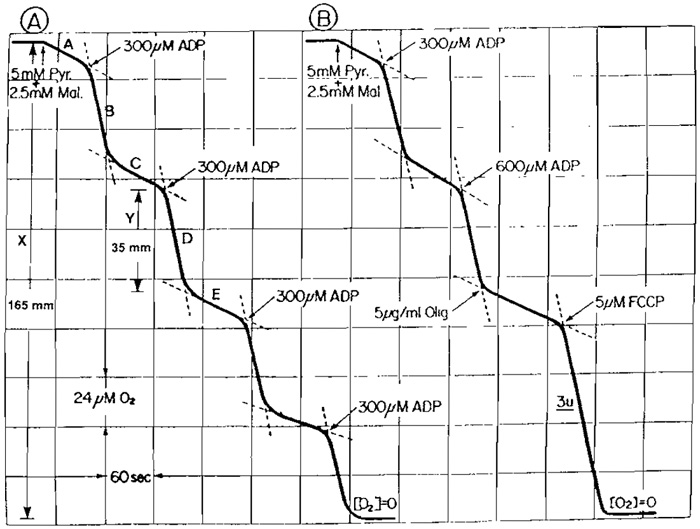 |
| FIGURE 2 Polarographic tracing of mouse skeletal muscle mitochondria oxidizing pyruvate plus malate. The reaction mixture consists of 150mM sucrose, 25 mM Tri-HCl, and 10mM phosphate, pH 7.5. Additions are as indicated. Volume, 1.0ml; temperature, 30°C. |
- It is imperative that glass redistilled water be used throughout all the experiments.
- A closed reaction vessel free of air bubbles and back diffusion of oxygen are essential for the success of polarographic assay of mitochondrial function.
- It is imperative that the excessive part of the polyethylene membrane, which covers the electrode after secured by a rubber O ring, be removed with a sharp blade, as the presence of excessive polyethylene membrane may trap materials and make cleaning difficult.
- All aqueous solutions of medium and reagent should be at neutral pH (i.e., 7.2-7.4). During the assays the temperature of the reaction mixture has to be maintained constant.
- When water-insoluble reagents are used, at the completion of the experiment, the reaction chamber needs to be cleaned thoroughly first with ethanol followed by distilled water. Contamination of either reagent and/or ethanol of the chamber will result in obscure data, which cannot be interpreted.
- Tarnish of the Ag/AgCl electrode can be removed by cleaning with a cotton-tipped swab dipped in 4N NH4OH aqueous solution followed by rinsing thoroughly with distilled water.
- The polarographic oxygen electrode technique is a convenient method for the determination of P/O ratio and RCI. However, its suitability is dependent on the quality of the mitochondrial preparations. Meaningful results can only be derived from tightly coupled mitochondrial preparations that are free from contamination by other cellular constituents. Consideration of the tissue constituents, i.e., lipid content, and properties will aid in the design of isolation and purification procedures. For instance, as compared to liver and heart, only a small portion of the total mass of skeletal muscle consists of mitochondria, with the bulk of the tissue being myofibrils. Additionally, skeletal muscle contains a relatively high content of Ca2+, which is capable of damaging mitochondria during the isolation process. Because of the high content of myofibril, the separation of mitochondria from other components is very difficult in nonelectrolyte medium (e.g., the isotonic sucrose used to isolate mitochondria from liver and heart). A similar difficulty results when attempts are made to isolate mitochondria from brain. Because of its high lipid content and high rate of aerobic metabolism, improper isolation may result in data fraught with artifact. In recent years, the polarographic technique has been widely used to evaluate the lesions of mitochondrial functions of skeletal muscle derived from patients suffering from mitochondrial myopathy. A reproducible technique that isolates tightly coupled, intact mitochondria is essential before statements regarding etiology can be made. Impairments in mitochondrial function may actually result from an artifact generated during the isolation procedure rather than a genuine impairment of mitochondrial function caused by the disease.
References
Chance, B., and Williams, G. R. (1955). Respiratory enzymes in oxidative phosphorylation I: Kinetics of oxygen utilization. J. Biol. Chem. 217, 383-393.
Davies, P. W., and Brink, E J. (1942). Microelecerodes for measuring local oxygen tension in animal tissues. Rev. Sci. Instr. 13, 524-533.
Jaworek, D., Gruber, W., and Bergmeyer, H. U. (1974). Adenosine-5'- diphosphate and adenosine-5'-monophosphate. In "Methods of Enzymatic Analysis," 2nd ed., Vol. 4, pp. 2027-2029.
Lardy, H. A., and Wellman, H. (1952). Oxidative phosphorylations: Role of inorganic phosphate and acceptor systems in control of metabolic rates. J. Biol. Chem. 195, 215-224.
Lee, C. P. (ed.) (1994). Molecular basis of mitochondrial pathology. Curr. Top. Bioenerg. 17, 254.
Lee, C. P., Sciamanna, M. A., and Peterson, P. L. (1993b). Intact brain mitochondria from a single animal: Preparation and properties. Methods Toxicol. 2, 41-50.
Sciamanna, M. A., and Lee, C. P. (1993). Ischemia/reperfusioninduced injury of forebrain mitochondria and prevention by ascorbate. Arch. Biochem. Biophys. 305, 215-224.
Sciamanna, M. A., Zinkel, J., Fabi, A. Y., and Lee, C. P. (1992). Ischemia injury to rat forebrain mitochondria and cellular calcium homeostasis. Biochim. Biophys. Acta 1134, 223-232.
Xiong, Y., Gu, Q., Peterson, P. L., Muizelaar J. P., and Lee C. P. (1997a). Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14, 23-34.
Xiong, Y., Peterson, P. L., and Lee, C. P. (1999). Effect of Nacetylcysteine on mitochondrial function following traumatic brain injury in rats. J. Neurotrauma 16, 1067-1082.
Xiong, Y., Peterson, P. L., Muzelaar, J. P., and Lee, C. P. (1997b). Amelioration of mitochondrial dysfunction by a novel antioxidant U- 101033E following traumatic brain injury. J. Neurotrauma 14, 907-917.
Xiong, Y., Peterson, P. L., Verweij, B. H., Vinas, E C., Muzelaar, J. P., and Lee, C. P. (1998). Mitochondrial dysfunction after experimental traumatic brain injury: Combined efficacy of SNX-111 and U-101033E. J. Neurotrauma 15, 531-544.




