Thyroid Tissue-Organotypic Culture Using a New Approach for Overcoming the Disadvantages of Conventional Organ Culture
The organ culture of thyroid tissue has been applied to the studies of thyroid biology (Bussolati et al., 1969; Cau et al., 1976; Young and Baker, 1982). However, the conventional organ culture system can not retain viable three-dimensional (3D) thyroid follicles containing both thyrocytes and C cells for a term long enough to investigate their biological behavior, as the tissue becomes necrotic progressively. Although the conventional method allows thyrocytes to grow out only in a monolayer from the tissue periphery placed on culture dishes, it cannot enable them to organize and maintain 3D follicles due to the lack of a 3D microenvironment of extracellular matrix (ECM) (Toda et al., 1996, 2001). Thyroid follicles in vivo are embedded in an interfollicular ECM, supported by a dense network of fenestrated capillaries (Fujita and Murakami, 1974). This suggests that both ECM and sufficient oxygen supply are important for the maintenance of follicular structure and function. By simulating this in vivo microenvironment of follicles, we have established a new organotypic culture using a 3D collagen gel culture of thyroid tissue fragments with improved oxygenation through air exposure (Toda et al., 2002). This system maintains very well the 3D follicle structures containing both thyrocytes and C cells for more than 1 month. Furthermore, the new follicle formation from preexisting follicles (mother folliclederived folliculogenesis) takes place actively in the peripheral zones of each tissue fragments in our system (Toda et al., 2003). We herein describe a useful tool for the long-term organ culture of thyroid tissue. In relation to our method, we propose a new approach to cell type-specific culture systems on the basis of in vivo microenvironments of various cell types.
Materials, reagents, and equipment are as follows: (1) 6-month-old porcine or human thyroid, (2) Eagle's MEM (EMEM, Cat. No. 05900, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan), (3) dispase I solution (bacterial neutral protease, 1000 protease U/ml in EMEM, Cat. No. GD 81020, Goudoh-Shusei, Tokyo, Japan), (4) Ham's F12 (Cat. No. 05910, Nissui Pharmaceutical Co., Ltd.), (5) fetal bovine serum (FBS, Cat. No. F9423, Lot No. 92K2301, Sigma Chemical Co., MO), (6) gentamicin (Gentamicin, Shering-Plough Co., Ltd., Osaka, Japan), (7) complete medium (Ham's F-12 medium supplemented with 10% FBS and 50µg/ml gentamicin), (8) acid-soluble type I collagen (Cellmatrix type I-A, Nitta Gelatin Co. Ltd., Osaka, Japan), (9) reconstructive buffer (2.2g NaHCO3 and 4.77g HEPES in 100ml 0.05M NaOH), (10) a special culture dish of which the bottom is made with nitrocellulose membrane (30 mm diameter, Millicell-CM, Cat. No. PICAM 3050, Millipore, Bedford, MA), (11) 90-mm-diameter bacterial dish (Cat. No. SH-2OS, Terumo Co., Ltd., Tokyo, Japan), and (12) stainless-steel mesh (840µm, Cat. No. Testing Sieve 840, Ikemoto Rikakogyo Co., Ltd., Tokyo, Japan). All materials, reagents, and equipment used in culture must be sterile.
A. Initial Preparation of Tissue for the Organotypic Culture of the Thyroid
- For porcine thyroid, tissues must be kept in icecold EMEM from the slaughterhouse to laboratory. Wash the tissue three times with ice-cold EMEM and remove connective and adipose tissues.
- For human thyroid, obtain tissues from surgical materials with permission and follow the same procedures as in step 1.
- For obtaining C cell-rich follicles, use tissue derived from only the middle to upper third of the lateral lobe of porcine and human thyroids, as C cells are mainly restricted to this area.
Steps
- Mince thyroid tissue into small fragments (~2mm) with sterile scissors.
- Transfer the tissue fragments (5g) into a 100-ml beaker containing 50 ml dispase I solution and incubate at 37 °C for 1 h.
- Remove the dispase I solution by filtrating the fluid through mesh (840 µm).
- Further mince the tissue remaining on the mesh into smaller pieces (~0.5 ram) with sterile scissors.
- Transfer the fragments into a 50ml-test tube, wash the tissue fragments with ice-cold EMEM by pipetting, and then centrifuge the tissue suspension at 186g for 5rain at room temperature. Repeat these procedures twice.
- The thyroid tissue fragments obtained as a pellet after the final centrifugation are subjected to the following organotypic culture.
In comparison with the conventional organ culture, our culture system is characterized by the following items. (1) Minced tissues are placed in a 3D collagen gel. (2) They are supplied sufficient oxygen through air exposure. The two conditions result in allowing the tissue fragments to situate under a 3D air-liquid (A-L) interface, but not under a submerged state. In this system, the tissues are kept moist and fed with the culture medium that percolated by capillary action from the medium-containing outer dish, through the acellular layer, and into the cellular layer (Toda et al., 2000, 2002, 2003). Likewise, the culture cells can be stimulated by various reagents added to the culture medium of the outer dish. In addition, exposing the cellular layer to various concentrations of oxygen permits the embedded cells to be supplied those of oxygen. Figure 1 illustrates our organotypic culture system.
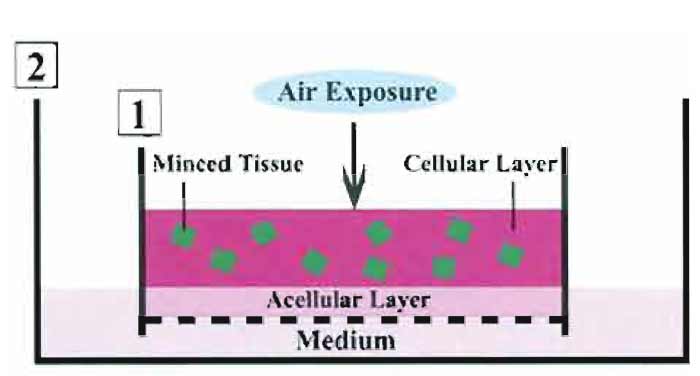 |
| FIGURE 1 Scheme of thyroid tissue-organotypic culture system. Minced tissues embedded in type I collagen gel (cellular layer) are placed on the acellular gel (acellular layer) in the inner dish (1). The inner dish (1) is put in the outer dish (2) with culture medium. In this way, tissues in the cellular layer are localized under air exposure-induced oxygenation. Tissues are kept moist and are fed by culture medium that percolates by capillary action from the medium-containing outer dish through the acellular layer and into the cellular layer. |
- Prepare the collagen gel solution as follows (Elsdale and Bard, 1972). First, mix 8 volumes of acidsoluble type I collagen with 1 volume of 10× concentrated Ham's F-12 medium by gently pipetting in a test tube. Second, add I volume of reconstructive buffer to the mixture and pipette it gently. Keep this mixture on ice.
- Place 2ml cold collagen gel solution in a special dish containing the nitrocellulose membrane and immediately warm the dish to 37 °C for at least 30min in a 5% CO2 incubator for gel formation. The collagen gel layer is called the acellular layer. Preparation of this layer should be completed before the beginning the steps in Section III,A.
- Pore 1 ml cold collagen solution into a test tube containing the tissue fragments obtained as a pellet. Then, gently and fully mix 1 ml cold collagen gel solution with the tissue fragments (a total of 0.5g). The initial amount of 5 g tissue results in the preparation of 10 culture dishes.
- Place 1ml collagen gel solution containing the fragments on the acellular layer and immediately warm the dish to 37°C for at least 30min in a 5% CO2 incubator to allow gel formation. The resultant overlayer is the cellular layer. The culture dish prepared in this way is referred to as the "inner" dish.
- After at least 30min, when the gel is fully firm, place the inner dish in a larger "outer" dish (90mm in diameter) containing 10ml complete medium.
- Place this culture assembly in a conventional culture incubator, thereby exposing the cellular layer to a humidified air atmosphere supplemented with 5% CO2 at 37°C. In this way, the tissue fragments are situated under a microenvironment that consists of both type I collagen and air exposure-induced oxygenation. We call this culture condition a 3D A-L interface.
Culture cells can be observed by phase-contrast microscopy. The collagen gel layer containing viable cells is scraped easily from the culture assembly. The layer can be treated similar to the various tissues resected from the body and used for analyzing the cellular behavior as follows. (1) The cellular layer gels are fixed in 4% formalin, processed routinely, and embedded in paraffin. The deparaffinized and frozen sections are applied easily to histochemistry, immunohistochemistry, and in situ hybridization. (2) To examine the fine structure of the cells, transmission electron microscopy is carried out using cellular layer gels fixed in 2.5% glutaraldehyde and prepared by a standard method. (3) Biochemical and genetic analyses of the cells can be carried out by the various methods described in this volume.
D. Examples of Thyroid Tissue-Organotypic Cultures
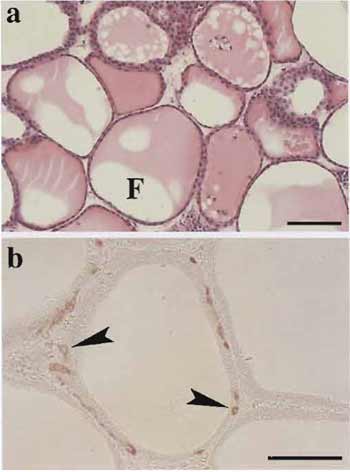 |
| FIGURE 2 Histology of thyroid tissues (a) and immunohistochemistry for calcitonin (b) in the organotypic culture. (a) At 40 days in culture, viable thyroid follicles enclosed by thyrocytes contain colloid substance in their lumens (F). (b) At 30 days in culture, thyroid follicles consisting of both thyrocytes and C cells (arrowheads) are clearly maintained. Scale bar: 100µm. |
In this system, viable 3D follicles within thyroid tissue fragments are maintained for more than 1 month. These follicle structures consist of thyrocytes and C cells with their specific differentiation (Fig. 2). In the tissue periphery, thyrocytes undergo actively growth and mother follicle-derived thyroid folliculogenesis. Likewise, isolated or clustered thyrocytes, which were localized in the tissue periphery at the starting time of the culture, reconstruct follicles. C cells show no proliferative ability and cannot grow even with the stimulation of various concentrations of free calcium. Most endothelial cells of capillaries disappear until 7 days in culture (for further details, see Toda et al., 1990, 1992, 1993, 1997, 2002, 2003).
In relation to our culture system, it seems that the time has come to reconsider conventional culture methods in a cell type-specific way. The microenvironments of many cell types of the body are subdivided mainly into the following three types. The first is that of parenchymal and stromal cell types of solid organs, e.g., thyroid, adrenal, and liver. The second is that of surface-lining cell types on which sufficient liquid is not overlayed, e.g., those of the skin, cornea, respiratory, and digestive tracts. The third is the microenvironment of surface-lining cell types on which enough fluid is overlayed, e.g., those of the cardiovascular system and cerebral ventricle. With respect to the first microenvironment, the following is our notion regarding that of the in vivo extravascular stroma by which various cell types are supported. The extravasucular space consists of ECM and tissue fluid percolated from blood vessels. The tissue fluid blended by nutrients and air molecules infiltrates into ECM and results in formation of the moist stroma. The moist microenvironment is different from the intravascular one, which has the sufficient liquid of blood. Our culture system seems to simulate simply the first microenvironment of extravascular stroma (Toda et al., 2001, 2002, 2003). Thus, our system is suitable for culturing various cell types other than surface-lining cell types.
On the basis of the in vivo microenvironment of various cell types, we propose the following three culture systems in a cell type-specific manner. (1) The usual monolayer culture under a submerged condition with enough medium is suitable for culturing the surface-lining cell types of endothelial cells, ependymocytes, and so on (Tokunaga et al., 1991). (2) The A-L interface culture is useful for culturing the surface-lining cell types of epidermis, cornea, respiratory, and digestive tracts (Nishimura et al., 1998; Yamada et al., 1999; Sugihara et al., 2001; Ootani et al., 2003). (3) A three-dimensional collagen gel culture with an A-L interface is suitable for culturing parenchymal and stromal cell types of solid organs (Toda et al., 2000, 2002, 2003).
The organ culture method described in this article is very simple and it is reproduced easily by beginners. The only one pitfall regarding our method is as follows: the collagenase solution should not be used to loosen ECM of thyroid tissue because the collagen gel layer is often digested by the remaining collagenase, even after washing the tissue fragments three times. Thus, dispase I solution is recommended.
References
Bussolati, G., Navone, R., Gasparri, G., and Monga, G. (1969). in vitro study of C (parafollicular) cells of dog thyroid in organ culture. Experimentia 25, 641-642.
Cau, P., Michel-Bechet, M., and Fayet, G. (1976). Morphogenesis of the thyroid follicles in vitro. Adv. Anat. Embry. Cell Biol. 52, 5-65. Elsdale, T., and Bard, J. (1972). Collagen substrata for studies on cell behavior. J. Cell Biol. 54, 626-637.
Nishimura, T., Toda, S., Mitsumoto, T., Oono, S., and Sugihara, H. (1998). Effects of hepatocyte growth factor, transforming growth factor-beta1 and epidermal growth factor on bovine corneal epithelial cells under epithelial-keratocyte interaction in reconstruction culture. Exp. Eye Res. 66, 105-116.
Ootani, A., Toda, S., Fujimoto, K., and Sugihara, H. (2003). Foveolar differentiation of mouse gastric mucosa in vitro. Am. J. Pathol. 162, 1905-1912.
Toda, S., Aoki, S., Suzuki, K., Koike, E., Ootani, A., Watanabe, K., Koike, N., and Sugihara, H. (2003). Thyrocytes, but not C cells, actively undergo growth and folliculogenesis at the periphery of thyroid tissue fragments in three-dimensional collagen gel culture. Cell Tissue Res. 312, 281-289.
Toda, S., Koike, N., and Sugihara, H. (2001). Thyrocyte integration, and thyroid folliculogenesis and tissue regeneration: Perspective for thyroid tissue engineering. Pathol. Int. 51,403-417.
Toda, S., Matsumura, S., Fujitani, N., Nishimura, T., Yonemitsu, N., and Sugihara, H. (1997). Transforming growth factor-J51 induces a mesenchyme-like cell shape without epithelial polarization in thyrocytes and inhibits thyroid folliculogenesis in collagen gel culture. Endocrinology 138, 5561-5575.
Toda, S., and Sugihara, H. (1996). Primary culture of the thyroid: Three-dimensional culture using extracellular matrix. In "Cell and Tissue Culture: Laboratory Procedures" (J. B. Griffiths, A. Doyle, and D. G. Newell, eds.), pp. 17B:2.1-12. Wiley, Baffins Lane, England.
Toda, S., Watanabe, K., Yokoi, E, Matsumura, S., Suzuki, K., Ootani, A., Aoki, S., Koike, N., and Sugihara, H. (2002). A new organotypic culture of thyroid tissue maintains three-dimensional follicles with C cells for a long term. Biochem. Biophys. Res. Commun. 294, 906-911.
Toda, S., Yokoi, E, Yamada, S., Yonemitsu, N., Nishimura, T., Watanabe, K., and Sugihara, H. (2000). Air exposure promotes fibroblast growth with increased expression of mitogen-activated protein kinase cascade. Biochem. Biophys. Res. Commun. 270, 961-966.
Toda, S., Yonemitsu, N., Hikichi, Y., Koike, N., and Sugihara, H. (1992). Differentiation of human thyroid follicle cells from normal subjects and Basedow's disease in three-dimensional collagen gel culture. Pathol. Res. Pract. 188, 874-882.
Toda, S., Yonemitsu, N., Minami, Y., and Sugihara, H. (1993). Plural cells organize thyroid follicles through aggregation and linkage in collagen gel culture of porcine follicle cells. Endocrinology 133, 914-920.
Tokunaga, O., Yamada, T., Fan, J. L., and Watanabe, T. (1991). Agerelated decline in prostacyclin synthesis by human aortic endothelial cells: Qualitative and quantitative analysis. Am. J. Pathol. 138, 941-949.
Yamada, S., Toda, S., Shin, T., and Sugihara, H. (1999). Effects of stromal fibroblasts and fat cells and an environmental factor air exposure on invasion of laryngeal carcinoma (HEp-2) cells in a collagen gel invasion assay system. Arch. Otolaryngol. Head Neck Surg. 125, 424-431.
Young, B. A., and Baker, T. G. (1982). The ultrastructure of rat thyroid glands under experimental conditions in organ culture. J. Anat. 135, 407-412.




