Tissue Microarrays
Tissue microarray (TMA) technology significantly facilitates and accelerates in situ analysis of tissues (Bubendorf et al., 1999; Kononen et al., 1998) as it allows the simultaneous analysis of up to 1000 tissue samples on a single microscope glass slide. In this method, minute tissue cylinders (typical diameter: 0.6mm) are removed from hundreds of different primary tumor blocks and subsequently brought into one empty "recipient" block. Paraffin-embedded tissues are optimal for TMA making, but frozen tissue samples can also be utilized (Simon and Sauter, 2002). Sections from such TMA blocks can be used for simultaneous in situ analysis of hundreds to thousands of tissue samples on the DNA, RNA, and protein level. The TMA technique has a number of distinct advantages as compared to the "sausage" block technique that has been suggested previously for analyzing multiple different tissues on one slide (Battifora, 1986). The regular arrangement of samples of identical shape and diameter on TMA sections greatly facilitates automated analysis and also orientation during manual analysis of staining. The cylindrical shape and the small diameter of the specimen taken out of the donor block maximize the number of samples that can be taken out of one donor block and minimize the tissue damage inferred to it. The latter is important for pathologists because they can give researchers access to their tissue material and at the same time retain their tissue blocks. Punched tissue blocks remain fully interpretable for all morphological and molecular analyses that may subsequently become necessary, provided that the number of punches is reasonably selected. Dozens of punches can be taken from one tumor without compromising interpretability (Bubendorf et al., 2001).
Manufacturing TMAs is a four-step process, including sample collection, preparation of recipient blocks, construction of TMA blocks, and sectioning. Material requirements and the laboratory procedures for all of these steps are described separately.
A. Materials and Instrumentation
Sample Collection
Standard routine histology microscope for review of tissue sections
Colored pens to mark representative areas on stained slides, e.g., red for tumor, blue for normal, and black for premalignant lesions
Sufficient working space especially for large-scale projects that require extensive sorting of thousands of slides and blocks (For frozen tissue TMA making, sufficient empty freezer space is needed.)
B. Preparing Recipient Blocks
1. Paraffin-Embedded and Frozen Tissue
Slotted processing/embedding cassettes for routine histology (Electron Microscopy Sciences Inc., PA, e.g., EMS Cat. No. 70070)
Stainless-steel base molds for processing/embedding systems (Electron Microscopy Sciences Inc., e.g., EMS Cat. No. 62510-30)
PEEL-A-WAY embedding paraffin pellets, melting point: 53-55°C (Polysciences Inc., PA, Cat. No. 19797)
Filter/filter paper (Schleicher & Schuell, Dassel, Germany; Faltenfilter diameter 185mm, Cat. No. 311647)
Oven for paraffin melting
3. Frozen Tissue Only
OCT Tissue-Tek compound embedding medium (Sakura BV, The Netherlands; Cat. No. 4583)
Dry ice
C. TMA Block Making
1. Paraffin-Embedded and Frozen Tissue
Tissue arrayer (currently there are two commercial vendors for tissue arrayers: http://www.beecherinstruments. com; http://www.chemicon.com) and supplies (extra needles, block holder). Several groups have introduced inexpensive modifications to the existing commercially available manual nonautomated arrayers, which improve performance markedly and facilitate frozen tissue arraying.
Premade empty recipient blocks (paraffin or frozen)
Illuminated magnifying lenses and supplies (e.g., Luxo U wave II/70, Cat. No. 27950, Luxo Inc., Switzerland) (optional)
2. Frozen Tissue Only
Dry ice to cool punching needles, tumor samples, and recipient block
D. TMA Block Sectioning
1. Paraffin-Embedded and Frozen Tissue
Boxes for slide storage
Refrigerator/freezer for slide storage
2. Paraffin-Embedded Tissue Only
Standard routine histology microtome and supplies (e.g., Leica SM2400, Leica Microsystems Inc., IL)
Sectioning Aid-System (Instrumedics Inc., NJ, Cat. No. PSA) containing ultraviolet curing lamp, adhesivecoated PSA slides, TPC solvent, TPC solvent can, hand roller, tape windows (optional)
Standard routine histology cryostat and supplies (e.g., Microm, HM560, Walldorf, Germany)
CryoJane consumables: adhesive-coated slides (Instrumedics Inc., Cat. No. CS4x, tape windows, Cat. No. TW) (optional)
III. PROCEDURES
A. Sample Collection
Although a device is needed to manufacture TMAs, it must be understood that most of the work (approximately 95%) is traditional histology work. Therefore, the use of automated tissue arrayers cannot accelerate TMA production. This preparatory work is similar to what is needed for traditional studies involving "large" tissue sections. The major difference is the number of tissues involved, which can be an order of magnitude higher in TMA studies than in traditional projects. The different tasks related to sample collection include the following.
- Exactly define the TMA to be made. Include normal tissues of the organ of interest and, if possible, of a selection of other organs as well.
- Generate a list of potentially suited tissues.
- Collect all slides from these tissues from the archive. In case of frozen tissues, representative slides of potential donor tissues may not be available and must be newly made.
- One pathologist must review all sections from all candidate specimens to select the optimal slide. If possible, tissues (especially in the case of tumors) should be reclassified during this review process according to current classification schemes. Tissue areas suited for subsequent punching should be marked. It is advisable to prepare a freshly HE-stained section if the actual block surface is not well reflected on the available stained section. Different colors are recommended for marking different areas on one section (e.g., red for tumor, black for carcinoma in situ, blue for normal tissue).
- Collect the required tissue blocks. These blocks and their corresponding marked slides must be matched and sorted together in the order of appearance on the TMA.
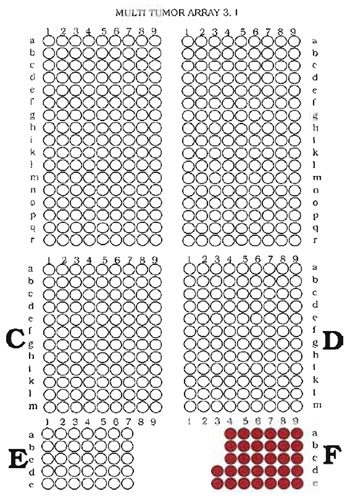
- Define the structure (outline) of the TMA and compose a file that contains the identification numbers of the tissues together with their locations and real coordinates (as they need to be selected on the arraying device). As a distance between the individual samples, 0.2mm is recommended for paraffinembedded materials and 0.4mm for frozen tissues if standard punching needles (diameter of 0.6mm) are used. To facilitate navigation on the TMA, arranging the tissues in multiple sections (e.g., quadrants) is recommended. The distance between the quadrants may be 0.8mm for paraffin-embedded materials and 1.0mm for frozen tissues. For unequivocal identification of individual samples on TMA slides, it is important to avoid a fully symmetrical TMA structure. In our laboratory, capitalized letters define quadrants, whereas small letters and numbers define the coordinates within these quadrants. Examples of a TMA structure (outline) and data file containing the necessary information for making a TMA are given in Fig. 1 and Table I.
 |
1. Paraffin-Embedded Tissue
In contrast to normal paraffin blocks, tissue microarray blocks are cut at room temperature. Therefore, a special type of paraffin is needed with a melting temperature between 53 and 55°C
("Peel-A-Way" paraffin, see Section IIA).
- Melt paraffin overnight at 60°C filtrate, and pour in a stainless-steel mold.
- Place a slotted plastic embedding cassette (as used in every histology laboratory) on top of the warm paraffin.
- Cool down recipient paraffin blocks for 2h at room temperature and for another 2hr at 4°C. Remove blocks from the mold. It is important not to cool down the paraffin on a cooling plate because of the risk of block damage.
- Quality check the recipient blocks to make sure that no air bubbles are included.
2. Frozen Tissue
Preparing recipient OCT blocks that are equally sized as paraffin recipient blocks ensures that the currently available tissue arrayers can be utilized for frozen TMA manufacturing.
- Place OCT into a standard cryomold and cool down over dry ice to form recipient blocks.
- Place an empty plastic biopsy embedding cassette on top of the freezing (but not yet completely frozen) OCT. This plastic cassette is subsequently filled with OCT.
- After slight thawing, remove the completely frozen recipient block from the cryomold. Immediately place the recipient block on dry ice or in a freezer until used.
Only if all this preparatory work has been done can a tissue-arraying device be employed. Using these manually operated devices, excellent TMAs can be produced in the hands of a talented and experienced person. However, optimal arrays can be expected only after a significant training period, including several hundred, if not a few thousand, punches. A patient and enduring personality as well as keen eyesight are important prerequisites for operators of manual tissue arrayers. Early generation automated tissue arrayers are available but these devices are expensive, do not accelerate the TMA manufacturing process, and cannot be used for making frozen TMAs.
1. Paraffin-Embedded Tissue
The TMA manufacturing process consists of five steps that are repeated for each sample placed on the TMA.
- Punch a hole into an empty recipient paraffin block.
- Remove and discard the wax cylinder from the needle used for recipient block punching.
- Remove a cylindrical sample from a donor paraffin block.
- Place the cylindrical tissue sample in the premade hole in the recipient block.
- Proceed to the new coordinates for the next tissue sample.
Exact positioning of the tip of the tissue cylinder at the level of the recipient block surface is crucial for the quality and the yield of the TMA block. Placing the tissue too deeply into the recipient block results in empty spots in the first sections taken from the TMA block. Positioning the tissue cylinder not deep enough causes empty spots in the last sections taken from this TMA. However, a too superficial location of the tissue cylinder is less problematic than a too deep position, as protruding tissue elements can, to some extent, be leveled out after finishing the punching process. The use of a magnifying lens facilitates precise deposition of samples, especially for beginners.
2. Frozen Tissue
The recipient block must always be surrounded with dry ice to prevent melting. Make sure that the micrometer screws are not covered by ice. Malfunctions occur if the temperature of these elements gets too low. Continuously add more ice as melting occurs. The TMA manufacturing process consists of five steps that are repeated for each sample placed on the TMA.
- Punch a hole into an empty recipient OTC array block.
- Remove and discard frozen OTC from the needle.
- Remove a cylindrical tissue sample (diameter 0.6mm; height 4-5 mm) from frozen tissues. For this purpose, the same needle is used as previously for making a hole into the recipient array block. Switching to a larger needle is not needed. It is important to keep the tissue in the needle frozen during the procedure, e.g., by precooling the needle with a piece of dry ice before punching and while dispensing the tissue core into the recipient block. Use forceps for holding ice cubes for cooling needles.
- Place the cylindrical tissue sample in the premade hole in the recipient OTC array block.
- Proceed to the new coordinates for the next tissue sample.
D. TMA Sectioning
Regular sections may be taken from frozen and paraffin-embedded TMA blocks using a standard microtome or cryostat. However, the more samples a TMA block contains, the more difficult regular cutting becomes. As a consequence, the number of slides of inadequate quality increases with the size of the TMA and, in turn, fewer sections from the TMA block can effectively be analyzed. Using a tape sectioning aid is therefore recommended.
The tape sectioning kit (Instrumedics) facilitates cutting and leads to highly regular nondistorted sections that are ideally suited for automated analysis. In addition, the tape system may prevent arrayed samples from floating off the slide if very harsh pretreatment methods are used. However, the sticky glued slides have the disadvantage of increased background signals between the tissue spots in some IHC analyses and of a slightly deteriorated morphology of some tissue samples because of small visible glue dots within the samples. The tissue samples themselves do not show increased nonspecific background in IHC. Use of the tape sectioning system is described next.
- Place an adhesive tape on top of the TMA block in the microtome immediately before cutting. Always use a hand roller in order to avoid bubbles.
- Cut section (usually 5 µm). The tissue slice is now adhering to the tape.
- Transfer the tissue slice on a special "glued" Instrumedics slide (use the hand roller for this purpose; stretching of the tissue in a water bath or on a heating plate is not necessary). It is important to store "glued" slides in darkness before usage. Adhering slide properties deteriorate rapidly under light exposure.
- Expose the slide (tissue on the bottom) to UV light for 35s in order to polymerize the glue on the slide and on the tape.
- Dip the slides into TPC solution (Instrumedics) at room temperature for 5-10s. The tape can then be removed gently from the glass slide. The tissue remains on the slide.
- Air dry slides at room temperature.
Slides, adhesive tape, and hand roller are optimally stored within the cryostat microtome. They can only be used if their temperature is identical to the temperature within the cryostat microtome.
- Place a precooled adhesive tape on top of the TMA block in the cryostat microtome using a precooled hand roller immediately before cutting.
- Cut section (usually 10-12µm). The tissue slide is now adhering to the tape.
- Transfer the tissue slice on a special "glued" slide using a hand roller.
- Put the slide on dry ice for 60s.
- Remove the tape gently (TPC solution is not required for removal of tape after ice pretreatment).
- Immediately place slides into freezer (-70°C).
TMAs are suited for all types of in situ analysis methods, including immunohistochemistry, fluorescence in situ hybridization (FISH), and RNA in situ hybridization. All protocols that can be used on large sections will also work on TMAs. Extended deparaffinization is recommended, e.g., xylene exposure for 60-120min. Examples of stained TMA sections are shown in Fig. 2. One of the most significant differences as compared to traditional large section studies is the high level of standardization that can be achieved in TMA experiments. All slides of one TMA study are usually incubated in one set of reagents, assuring absolutely identical concentrations, temperatures, and incubation times. Other minor variables that may have an impact on the outcome of in situ analyses such as the age of a slide (time between sectioning and use) or section thickness are also fully standardized if all tissues of one study are located on the same TMA section. As a result of this unprecedented standardization within each experiment, intralaboratory variations may occur if experiments are repeated under slightly different conditions. Significant differences that were observed after using TMA sections stored for different time spans may represent just one example of how minor variables can significantly affect the results of molecular in situ analyses (M. Mirlacher et al., unpublished observations, 2002).
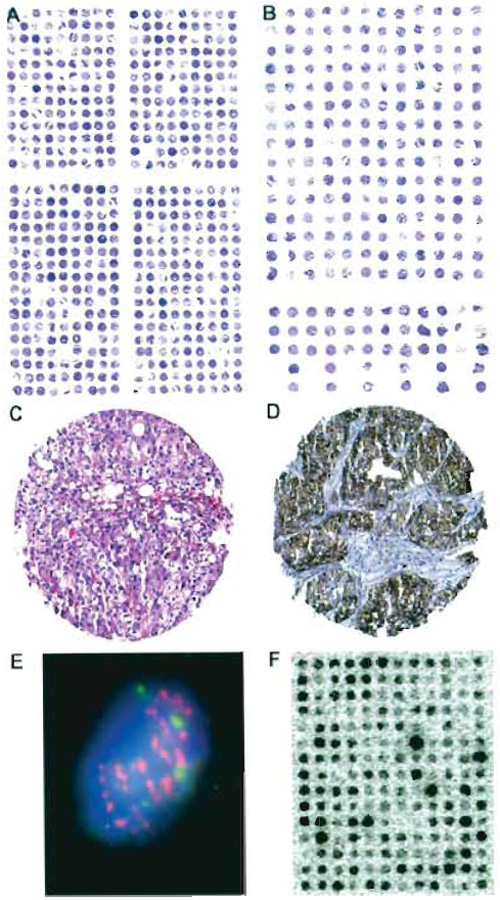 |
| FIGURE 2 Examples of stained tissue sections. Hematoxylin and eosin (H&E)-stained sections of (A) a TMA from formalin-fixed, paraffin-embedded tissues containing 540 tissue spots and (B) a TMA from frozen tissue containing 228 tissue spots. Each tissue spot measures 0.6 mm in diameter. Missing samples result from the sectioning/staining process or indicate samples that are already exhausted. Note that the spot-to-spot distance is larger on the frozen TMA as compared to the paraffin TMA. (C) Magnification of a H&E-stained 0.6-mm tissue spot of a bladder carcinoma. (D) Immunohistochemistry against the EGFR protein in a pharynx cancer sample using the DAKO HercepTest. (E) FISH analysis of centromere 7 (green signals) and the EGFR gene (red spots) in cell nuclei (blue staining) of a tissue spot (630x). The high number of EGFR signals indicates gene amplification. (F) RNA in situ hybridization on a frozen TMA made from breast cancer tissues. A radioactively labeled oligonucleotide was used as a probe against mRNA of a lipoprotein-binding protein. The black staining intensity indicates the level of mRNA in each tissue spot. |
V. TROUBLESHOOTING
For a description of problems, possible causes, and remedies, see Table II.
| TABLE II Troubleshooting | |||||||
| Problem | Possible cause | Paraffin embedded | Frozen | ||||
| Missing tissue samples on first series of TMA sections taken from TMA blocks (same samples missing on all sections) | Tissue cores placed too deeply into the recipient block | As long as the TMA making is not completed, a too deeply placed tissue core can be removed using the thinner needle. Then another sample taken from the donor block can be used as a replacement | Removal of the too deeply positioned tissue is not recommended. Consider adding an additional sample of this tissue to the TMA block | ||||
| Missing tissue samples on deeper sections taken from TMA blocks (same samples missing on all sections) | Tissue cores were not pushed into the recipient block deeply enough | Careful leveling out of the TMA block surface after finishing the punching process and placing the TMA block in an oven (10 min 40°C). | Careful leveling out of the TMA block surface after finishing the punching by gently pressing an empty glass slide on the surface (Do not use oven!) | ||||
| Tissue cores were too short | Check tissue blocks for presence of sufficient tumor material before final block selection | ||||||
| Missing tissue samples on deeper sections taken of TMA blocks (different samples missing on different sections) | Samples float off the slide during harsh pretreatments | Use adhesive-coated slides | |||||
| Samples come off the slide during the sectioning process | Avoid air bubbles between block and tape and between tape and adhesivecoated slide by using the hand roller. Slides and tapes may have irregular quality; it is important to check the material quality before use. Poor quality slides have inhomogeneous glue distribution | ||||||
| Some tissues on the TMA section are very thin and partially destroyed | Tissue damage incurred during removal of the adhesive tape | Do not cut sections thinner than 5µm. Exact timing of the tape removal process | Do not cut sections thinner than 10µm. | ||||
| Quality control of the unstained TMA slides under the microscope before use | Quality control of the unstained TMA slides under the microscope before use | ||||||
| Tissue on TMA section is not representative | Wrong area punched | Correct marking of areas on the HE-stained section that can be easily found on the block | |||||
| Careful selection of the marked area for punching | |||||||
| Irregular distribution of samples on the TMA block | Needle is bent | Change needle | To some extent irregular sample arrangement must be accepted | ||||
| Changing the needle improves the regularity of the TMA | |||||||
| Complete frozen TMA within 1 working day | |||||||
| Tissue cylinder cannot be removed from donor block | Needle is blunt | Change needle | Change needle | ||||
| Paraffin cylinder cannot be removed from recipient block | Needle is blunt | Change needle | Change needle | ||||
| Array block protrudes in the center ("hillock formation" in paraffin arrays) | Premade holes in the TMA recipient block are too short | Make deeper holes | |||||
| Too small a distance between tissue cylinders | Select sufficiently large distance between different cylinders | ||||||
| Careful leveling out of the TMA block surface after finishing the punching process and placing the TMA block in an oven (10min 40°C). | |||||||
| Paraffin recipient blocks crashes | Samples placed too closely to the border of the block | Select a distance of at least 3 mm between recipient block border and first tissue sample | |||||
| Use larger recipient blocks | |||||||
| Tissue core cannot be placed into the recipient block | Paraffin: needles are not aligned properly | Align needles (see manual) |
|||||
| Frozen: tissue melts during the punching process | Carefully cool the needle before and during the punching process | ||||||
| Micrometer screw malfunction during arraying | Battery exhausted | Change battery | Change battery | ||||
| Frozen: Dry ice is covering micrometer screw | Remove dry ice from micrometer screw | ||||||
Battifora, H. (1986). The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab. Invest. 55, 244-248.
Bremnes, R. M., Veve, R., Gabrielson, E., Hirsch, F. R., Baron, A., Bemis, L., Gemmill, R. M., Drab kin, H. A., and Franklin, W. A. (2002). High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J. Clin. Oncol. 20, 2417-2428.
Bubendorf, L., Kolmer, M., Kononen, J., Koivisto, P., Mousses, S., Chen, Y., Mahlamäiki, E., Schraml, P., Moch, H., Willi, N., Elkahlhoun, A., Pretlow, T., Gasser, T., Mihatsch, M., Sauter, G., and Kallioniemi, O. (1999a). Molecular mechanisms of hormone therapy failure in human prostate cancer analyzed by a combination of cDNA and tissue microarrays. J. Natl. Cancer Inst. 91, 1758-1764.
Bubendorf, L., Kononen, J., Koivisto, P., Schraml, P., Moch, H., Gasser, T., Willi, N., Mihatsch, M., Sauter, G., and Kallioniemi, O. (1999b). Survey of gene amplifications during prostate cancer progression by high-throughput fluorescence in situ hybridization on tissue microarrays. Cancer Res. 59, 803-806.
Bubendorf, L., Nocito, A., Moch, H., and Sauter, G. (2001). Tissue microarray (TMA) technology: Miniaturized pathology archives for high-throughput in situ studies. J. Pathol. 195, 72-79.
Fejzo, M. S., and Slamon, D. J. (2001). Frozen tumor tissue microarray technology for analysis of tumor RNA, DNA, and proteins. Am. J. Pathol. 159, 1645-1650.
Kononen, J., Bubendorf, L., Kallioniemi, A., Bäirlund, M., Schraml, P., Leighton, S., Torhorst, J., Mihatsch, M., Sauter, G., and Kallioniemi, O. (1998). Tissue microarrays for high-throughput molecular profiling of hundreds of specimens. Nature Med. 4, 844-847.
Schraml, P., Kononen, J., Bubendorf, L., Moch, H., Bissig, H., Nocito, A., Mihatsch, M. J., Kallioniemi, O. P., and Sauter, G. (1999). Tissue microarrays for gene amplification surveys in many different tumor types. Clin. Cancer Res. 5, 1966-1975.
Simon, R., and Sauter, G. (2002). Tissue microarrays for miniaturized high-throughput molecular profiling of tumors. Exp. Hematol. 30, 1365-1372.
Simon, R., Struckmann, K., Schraml, P., Wagner, U., Forster, T., Moch, H., Fijan, A., Bruderer, J., Wilber, K., Mihatsch, M. J., Gasser, T., and Sauter, G. (2002). Amplification pattern of 12q13-q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene 21, 2476-2483.
Torhorst, J., Bucher, C., Kononen, J., Haas, P., Schraml, P., Zuber, M., Köchli, O., Mross, F., Dieterich, H., Moch, H., Mihatsch, M., Kallioniemi, O., and Sauter, G. (2001). Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am. J. Pathol. 159, 2249-2256.