Viable Hybrids between Adherent Cells: Generation, Yield Improvement, and Analysis
Somatic cell hybridization was discovered and introduced by Barski et al. (1960) and Sorieul and Ephrussi (1961). This technique allows one to examine the result of introducing various genomes in different functional states and from different species into the same cell. Hybrid cells have been widely used in various fields (genetics, cell biology, tumour biology, virology) and the most famous hybrids are hybridomas.
One important application of somatic cell hybridization is chromosomal gene assignment. Breeding analysis, which is effective for this purpose in lower animals and plants, is too slow in mammals (even in mice the generation time is about 3 months) and is impossible in humans. Gene mapping techniques based on somatic cell genetics have been central to the study of human genetics. In 1968, only two genes had been mapped to specific autosomes, and a decade later this number had risen to 300, mostly using human-rodent somatic cell hybrids. Such hybrids present the advantage to retain only a few human chromosomes and they are now currently used as donor cells in irradiation and fusion gene transfer (IFGT) experiments for constructing detailed genetic maps (Walter and Goodfellow, 1993).
Somatic cell hybridization has not only shown that tissue-specific genes are regulated by trans-acting factors, but has provided strong evidence for the existence of tumour suppressor genes (Anderson and Stanbridge, 1993). Cell fusion experiments have also demonstrated that cellular senescence is a dominant active process and that several genes or genes pathways are implicated in the senescence program (Goletz et al., 1994).
Spontaneous fusion of cells in culture occurs at a very low frequency. To obtain hybrid cells, inactivated Sendai virus or, more commonly, polyethylene glycol (PEG), which was introduced by Pontecorvo (1976), is used as the fusogen. Cell hybridization has also been performed by electrofusion on filters (Ramos et al., 2002). The inital products of fusion contain within a common cytoplasm two or more distinct nuclei from one single parent (homokaryons) or from both (heterokaryons). Only a very small proportion of these polykaryons will progress to nuclear fusion and then through mitosis. Moreover, the first divisions of the heterokaryons and of their daughter cells often fail because of abnormalities of the mitotic spindle and abnormal chromosome movements. The formation of viable hybrids from heterokaryons is thus a rare event, and the use of selective methods that favour the survival of the hybrids at the expense of the parental cells is often a requisite. These selective methods are also necessary because hybrid cells often grow more slowly than parental cells and are rapidly overgrown by parental cells.
The best known of such methods is the application of hypoxanthine + aminopterin + thymidine (HAT) selection (Littlefield, 1964) for the fusion of cells deficient in hypoxanthine guanosine phosphoryl transferase (HGPRT-) with cells deficient in thymidine kinase (TK-), but different combinations of selectable markers can be used (Hooper, 1985), provided that the two selective systems do not interfere. If the lines that are fused have no selective markers, a good strategy is to select sequentially for HGPRT deficiency (thioguanine resistance) and ouabain resistance in one parental cell line. Then this marked cell line may be fused with any unmarked cell line and hybrids selected in HAT + ouabain (Jha and Ozer, 1976). Other couples of selective markers, such as TK deficiency (5-bromo- 2'deoxyuridine resistance) and neomycine resistance, can also be used. For producing primate-rodent hybrids, the selection of an HGPRT- rodent parent is sufficient because rodent cells are more resistant to ouabain than primate cells. Moreover, hybrid cells can also be isolated on the basis of their size, morphology, growth parameters, and DNA content.
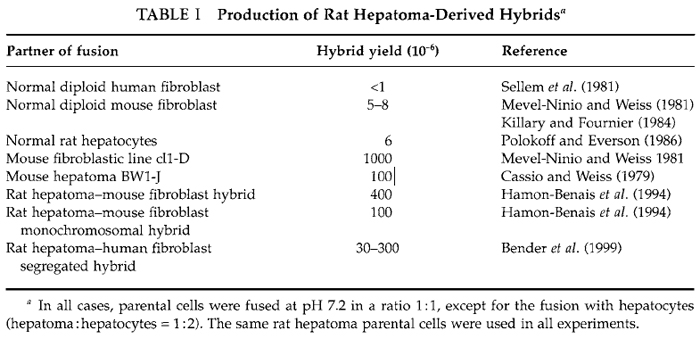
Whatever the method used to isolate hybrids, the most important is to optimize the fusion conditions in order to obtain a number of viable hybrids as high as possible. In the best cases the fusion of several millions of parental cells leads to the formation of only a few hundred hybrids and often the yield of viable hybrids is much lower, as illustrated in Table I for hepatomaderived hybrids. The protocol described here has been used routinely to produce large amounts of hybrid clones between differentiated rat hepatoma cells and various cells of different histogenetic origin and of different species, particularly mouse and human fibroblasts (Mevel-Ninio and Weiss, 1981; Sellem et al., 1981). Moreover, some of the hybrids obtained have been used themselves as partners of fusion and new hybrids were generated successfully using exactly the same method (Bender et al., 1999; Hamon-Benais et al., 1994). The most important parameters in fusion experiments are the yield of viable and growing hybrids and their stability. Thus it is recommended to define optimal fusion conditions and to vary different parameters, particularly the ratio of parental cells, for improving the yield. It is also recommended to analyze regularly hybrid clones for their phenotype and chromosomal content.
 |
II. MATERIALS
PEG 1000 ultrapure (Merck, Cat. No. 9729)
Trypsin (pig pancreas; United States Bioch. Corp., Cleveland, OH, Cat. No. 22715)
Complete growth medium (available from local suppliers)
Serum-free growth medium (available from local suppliers)
Selective complete growth medium (available from local suppliers)
35- and 50-mm tissue culture dishes (Falcon, Cat. No. 3001, 3002)
15-ml tube (Falcon, Cat. No. 352099)
22 × 22-mm sterile glass coverslips
A. Before Fusion
Solutions
- 50% PEG (for fusion): Autoclave PEG 1000. This both liquifies and sterilizes the PEG. Cool it to 50°C and mix with an equal volume of sterile serum-free medium prewarmed for a short period at 50°C. Adjust, if necessary, to the desired pH with 1.0 M NaOH (range of pH generally used is 7.2-7.9). This solution can be stored at 4°C for up to 2 weeks.
- 0.25 and 0.05% trypsin (to detach fusion products): To make 100ml of solution, solubilize 0.8g of NaCl, 0.04g of KCl, 0.058g of NaHCO3, 0.1g dextrose, and 0.25g (0.25%) or 0.05g (0.05%) of trypsin. Complete to 100ml distilled water. Incubate at 37°C for 1-2h. Sterilize by filtration and store at 4°C (rapid use) or -20°C.
Step
Grow parental cells in nonselective medium for a short period.
Steps
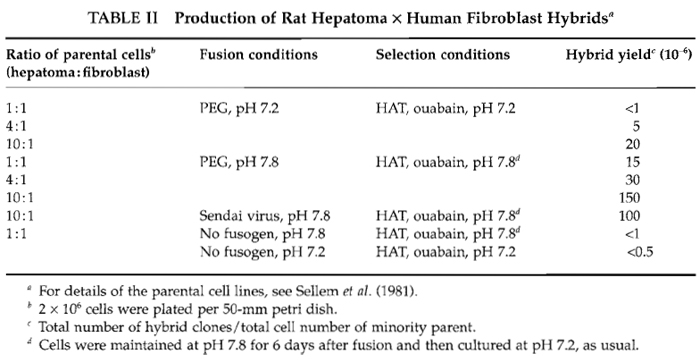
- Inoculate the mixture of parental cells to be fused into several 50mm tissue culture dishes containing complete (nonselective) growth medium. The total number of cells has to be adjusted to occupy all the dish surface, such that the fusion will be done on cells that are in close contact. For 50mm petri dishes, the total cell number could vary from 5 × 105 to 4 × 106 depending on the density at confluence of the cell lines used. Although equal numbers of parental cells are generally recommended, use different ratios of parental cells (see Table II and Section IV).
- Incubate the mixed cultures a few hours (4h to overnight). This allows the cells to adhere to the support and to establish contacts with neighbors.
- Warm the serum-free medium and the 50% PEG solution to 37°C.
- Remove the medium thoroughly from the culture and wash once with 5ml of serum-free medium.
- Add gently 3.0ml of PEG 50% all over the cell layer.
- After 45 s aspirate the PEG.
- Exactly I min after PEG treatment, add 5ml of serum-free medium.
- Aspirate half the medium and add 2.5ml of serum-free medium.
- Repeat step 8 four times.
- Aspirate all the medium.
- Add 5ml of complete growth medium and let the cells recover for at least 2h and no more than 12h.
Notes: Steps 4 to 11 must be done dish per dish. Some control dishes must be included. They will be treated as the others except that the PEG solution will be replaced by serum-free medium.
Steps
- Aspirate the medium and add 3ml of 0.25% trypsin per dish.
- As soon as the cell layer begins to detach, add 3 ml of 0.05% trypsin and detach the cells by repeated pipetting.
- Add the cell suspension in a 15-ml tube containing 2ml of complete growth medium (to arrest the trypsin action). If necessary, rinse the dish with 2ml medium and add this medium to the tube containing the cells.
- Centrifuge the cells at 500g (1500rpm) for 5min at room temperature.
- Resuspend thoroughly the cell pellet in complete growth medium and count the cell number in a hemacytometer. Using the described protocol, generally at least 80% of the cells are recovered after fusion.
- Pool, if necessary, cells recovered from identical dishes and inoculate different numbers of cells (103-106) in either culture dishes or on 22-mm glass coverslips (in 35-mm dishes).
- Incubate the cells at least overnight (eventually a few days) in complete growth medium before adding selective medium that will kill the parental cells and let the hybrid cells survive.
- At regular intervals, watch for the appearance of growing hybrid colonies. Count their number in dishes or coverslips that contain well-isolated colonies. From this number the yield of growing hybrids can be calculated. For one fusion, this yield is equal to the total number of hybrid clones obtained divided by the total cell number of the minority parent engaged in the fusion.
- Use dishes that contain a small number of colonies to isolate independent hybrid clones (one per dish will be scraped, subcultured, characterized, as soon as possible, frozen, and recharacterized). From dishes that contain a lot of colonies, if this situation arises, hybrid cell populations can be obtained in mass and studied rapidly.
- Use glass coverslips to control by cytogenetic methods the hybrid nature of the clones and to test if their chromosomal content is stable with time in culture. The karyotyping in situ method, described by Worton and Duff (1979), is highly recommended. This method is easy, of general application for adherent cells, and can be performed on small colonies even a few generations after fusion (Fig. 1). Glass coverslips can also be used for phenotypic characterization of hybrid clones.
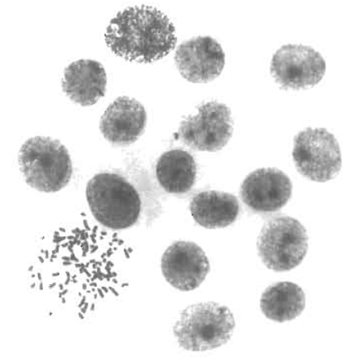 |
| FIGURE 1 Karyotypic analysis of emerging hybrids. The in situ karyotyping method (Worton and Duff, 1979) was performed on rat hepatoma-derived hybrid colonies 8 days after fusion. The colony shown was composed of 17 cells, 1 of which was in metaphase. This metaphase contains 100 chromosomes, corresponding to the expected sum of the mean chromosome number of each parent (46 and 52, respectively). |
The production of hybrid clones in large amounts depends greatly on the parental cells (cell type and growth capacity) and on the fusion conditions. These two points are illustrated in Tables I and II for rat hepatoma-derived hybrids. The frequency of occurrence of hybrids between rat hepatoma and normal fibroblast was particularly low compared to other hepatoma-derived hybrids (Table I). Consequently, various fusion conditions were tested. The use of unbalanced ratios of parental cells is one of the most important parameters to improve the hybrid yield (Table II). Therefore, to save time and to obtain the highest number of hybrid cells, it is recommended to fuse parental cells in different ratios.
Mixed parental cell populations that have not been treated by PEG can give rise to colonies that grow in selective medium at low frequency. These colonies could be either spontaneous hybrids or revertants from parental cells. The isolation of revertants is one of the most common difficulties that may arise in selecting hybrids. Therefore the hybrid nature of the cells selected has to be verified by checking their chromosomal content.
 |
- Some PEG preparations produce enormous lethality, whereas ultrapure PEG from Merck results in acceptable levels of lethality (generally below 20%).
- Detach and dissociate very carefully cells after fusion (avoid aggregates).
- Because it is impossible to predict if hybrid clones will be produced with a high yield or not, once fusion has been performed and the products of fusion detached, inoculate them at different concentrations (that could cover a 1000× range) such that well-isolated hybrids could be obtained even if the yield varies from 10-6 to 10-3.
- A lower number of hybrid clones is obtained if the cells are not detached after fusion.
- A lower number of hybrid clones is obtained if the mixture of parental cells is fused in suspension. This result is due to the fact that the relative proportion of binucleate heterokaryons is higher in monolayer fusion, whereas suspension fusion favours the formation of giant heterokaryons that die soon after fusion. Hence, there is no reason and no advantage to fuse adherent cells in suspension as often recommended (except if one of the parent grows in suspension).
Acknowledgments
I thank M. C. Weiss for training in cell culture and C. H. Sellem and C. Hamon-Benais for the illustrations.
Anderson, M. J., and Stanbridge, E. J. (1993). Tumor suppressor genes studied by cell hybridization and chromosome transfer. FASEB J. 7, 826-833.
Barski, G., Sorieul, S., and Cornefert, E (1960). Production dans des cultures in vitro de deux souches cellulaires en association, de cellules de caractére "hybride". C. R. Acad. Sci. Paris 251, 1825-1827.
Bender, V., Bravo, P., Decaens, C., and Cassio, D. (1999). The structural and functional polarity of the hepatic human-rat hybrid WIF-B is a stable and dominant trait. Hepatology 30, 1002-1010.
Cassio, D., and Weiss, M. C. (1979). Expression of fetal and neonatal hepatic functions by mouse hepatoma-rat hepatoma hybrids. Som. Cell Genet. 5, 719-738.
Goletz, T. J., Smith, J. R., and Pereira-Smith, O. M. (1994). Molecular genetic approaches to the study of cellular senescence. Cold Spring Harb. Symp. Quant. Biol. LIX, 59-66.
Hamon-Benais, C., Delagebeaudeuf, C., Jeremiah, S., Lecoq, O., and Cassio, D. (1994). Efficiency of a specific albumin extinguisher locus in monochromosomal hepatoma hybrids. Exp. Cell Res. 213, 295-304.
Jha, K. K., and Ozer, H. L. (1976). Expression of transformation in cell hybrids. I. Isolation and application of density-inhibited Balb/3T3 cells deficient in hypoxanthine phosphoribosyl transferase and resistant to ouabain. Som. Cell Genet. 2, 215-233.
Killary, A. M., and Fournier, R. E. K. (1984). A genetic analysis of extinction: Trans-dominant loci regulate expression of liverspecific traits in hepatoma-hybrid cells. Cell 38, 523-534.
Littlefield, J. W. (1964). Selection of hybrid from matings of fibroblasts in vitro and their presumed recombinants. Science 145, 709-710.
Mevel-Ninio, M., and Weiss, M. C. (1981). Immunofluorescence analysis of the time-course of extinction, reexpression and activation of albumin production in rat hepatoma-mouse fibroblast heterokaryons and hybrids. J. Cell Biol. 90, 339-350.
Polokoff, M. A., and Everson, G. T. (1986). Hepatocyte-hepatoma cell hybrids: Characterization and demonstration of bile acid synthesis. J. Biol. Chem. 261, 4085--4089.
Ramos, C., Bonenfant, D., and Teissie, J. (2002). Cell hybridization by electrofusion on filters. Anal. Biochem. 302, 213-219.
Sellem, C. H., Cassio, D., and Weiss, M. C. (1981). No extinction of tyrosine aminotransferase inducibility in rat hepatoma-human fibroblast hybrids containing the human x chromosome. Cytogenet. Cell Genet. 30, 47-49.
Sorieul, S., and Ephrussi, B. (1961). Karyological demonstration of hybridization of mammalian cells in vitro. Nature (Lond.) 190, 653-654.
Walter, M. A., and Goodfellow, P. N. (1993). Radiation hybrids: Irradiation and fusion gene transfer. Trends Genet. 9, 352-356.
Worton, R. G., and Duff, C. (1979). Karyotyping. Methods Enzymol. 58, 322-344.
Suggested Reading
Harris, H. (1995). "The Cells of the Body: A History of Somatic Cell Genetics." Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Ringertz, N. R., and Savage, R. E., (1976). "Cell Hybrids." Academic Press, London.
Zallen, D. T., and Burian, R. M. (1992). On the beginnings of somatic cell hybridization: Boris EPHRUSSI and chromosome transplantation. Genetics 132, 1-8.




