Vital Staining of Cells with Fluorescent Lipids
Although a number of fluorescent lipid analogs are now available, only a limited set of the molecules are frequently employed for cell biology. Because most of these molecules are less hydrophobic than naturally occurring counterparts, they are easily partitioned to cell membranes when added to the medium. This property and bulk fluorophore alter both physical and biological properties of the lipids. Therefore, the intracellular behavior of fluorescent lipids is not always the same as that of endogenous lipids. In this context, the results need to be evaluated carefully. However, together with appropriate control, fluorescent lipids are very useful tools to follow dynamics of membrane lipids.
II. MATERIALS AND INSTRUMENTATIONS
6-((N- (7-nitrobenz-2-oxa- 1,3-diazol-4-yl) amino) hexanoyl)sphingosylphosphorylcholine (C6-NBD-SM, MW 740.88, Cat. No. N3524), 6-((N-(7-nitrobenz-2- oxa-l,3-diazol-4-yl)amino)hexanoyl)sphingosine (C6- NBD-Cer, MW 575.75, Cat. No. Nl154) and N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indaene-3-pentanoyl)sphingosine (C5-BODIPY-Cer, MW 601.63, Cat. No. D3521) are from Molecular Probes. 12- ((N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)dodecanoyl) sphingosine (C12-NBD-Cer, MW 659.90, Cat. No. N9158) is from Sigma. 1-Palmitoyl-2-(6-((7-nitro-2- 1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sn-glycero-3-phospho-L-serine (ammonium salt) (C6-NBD-PS, MW 790.85, Cat. No. 810192) is obtained from Avanti. Structures of fluorescent lipids are summarized in Fig. 1.
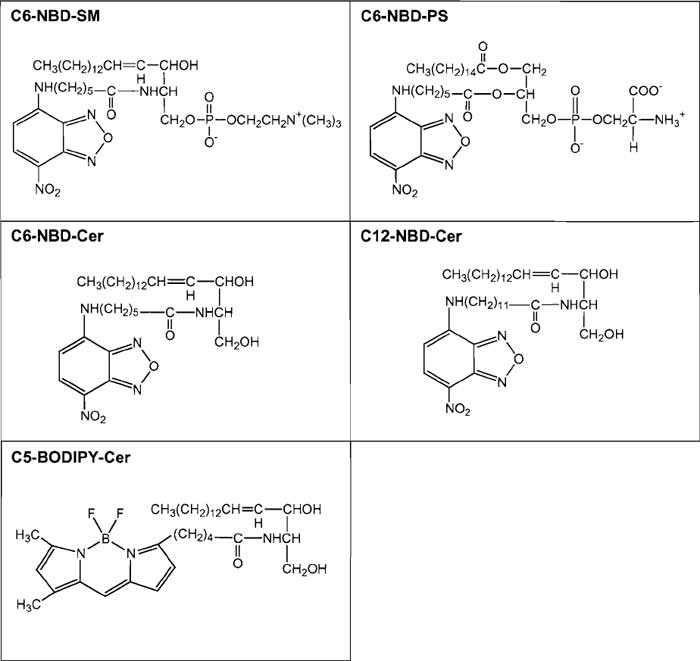 |
| FIGURE 1 Structures of fluorescent lipids described in the text. |
This procedure describes the methods used to follow the fate of fluorescent lipids inserted into the outer leaflet of the plasma membranes. The fate of fluorescent lipids is different depending on the hydrophilic head group of the lipids.
Solutions of Fluorescent Lipids
Stock solutions (1 mg/ml) of fluorescent lipids are prepared by dissolving C6-NBD-PS in chloroform and C6-NBD-SM in ethanol. The chloroform stock solution should be kept in screw-capped (Teflon-lined) glass tubes. The ethanol solution can be stored in either glass or plastic tubes. Stock solutions are stored at -20°C. An aliquot of a stock lipid solution is dried, first under a stream of nitrogen and then in vacuo. The dried lipid is then dissolved in DMEM to give final concentration 4µM. The solution is vortexed and kept at 4°C before use.
- Grow HeLa cells in duplicate on 35-mm glassbottom culture dishes for 1-2 days to reach 50-80% confluency.
- Wash cells twice with ice-cold DMEM.
- Incubate cells for 30min at 4°C with 1 ml DMEM containing 4 µM C6-NBD-SM or C6-NBD-PS.
- Wash one dish twice with ice-cold DMEM F-12 and then add 1 ml ice-cold DMEM F-12. Record fluorescence images under a confocal microscope (Fig. 2, "0 min"). 5. Wash the other dish twice with ice-cold DMEM and then add 1 ml prewarmed (37°C) DMEM.
- Incubate cells for 30min at 37°C in a CO2 incubator.
- Wash cells twice with DMEM F-12 at room temperature.
- Add 1 ml DMEM F-12 and observe cells under a microscope (Fig. 2, "30min").
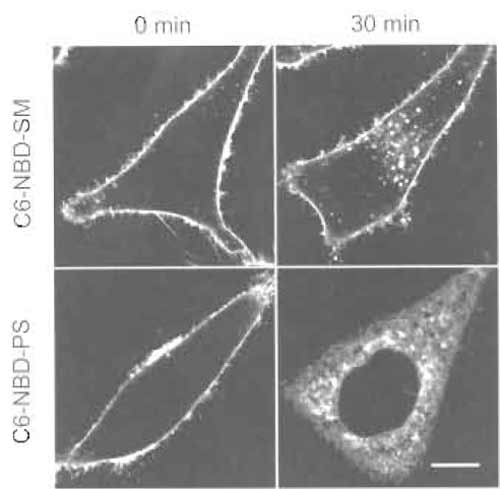 |
| FIGURE 2 HeLa cells labeled with C6-NBD-SM or C6-NBD-PS for 30min at 4°C (0 min) or 4°C treatment followed by a 30-min incubation at 37°C (30min). Bar: 10µm. |
C6-NBD-SM (Koval and Pagano, 1989) and C6- NBD-PS (Martin and Pagano, 1987) were first introduced by Pagano and co-workers. At low temperature (2 or 4°C), both fluorescent lipids are transported from the medium to the outer leaflet of the plasma membranes of cultured cells via spontaneous transfer of the lipids. Warming up of cells induces ATP-dependent transbilayer movement (flip-flop) of C6-NBD-PS (Martin and Pagano, 1987). In mammalian cells, the energy-dependent transbilayer movement of lipids is observed only with amino phospholipids such as phosphatidylserine and phosphatidylethanolamine (Devaux et al., 2002). Flip-flop is observed as low as 7°C. Once internalized, C6-NBD-PS is transported spontaneously to intracellular membranes. Unlike C6- NBD-PS, C6-NBD-SM stays on the outer leaflet of the plasma membrane and is internalized by endocytosis. It is postulated that C6-NBD-SM is a marker for the bulk flow of membranes after endocytosis (Mayor et al., 1993). C6-NBD-SM is also used together with C6- NBD-labeled glycolipids to study polarized lipid transport in hepatoma cells (Maier et al., 2002). Because DMEM F-12 contains HEPES, the pH keeps constant during incubation outside the CO2 incubator. We use phenol red-free DMEM F-12 when we take images under a microscope. BODIPY-labeled lipids (Pagano and Chen, 1998) and pyrene-labeled lipids (Somerharju, 2002) are also used for the similar purpose. The fluorescent cholesterol analog dihydroergosterol is introduced to follow the fate of cell surface cholesterol (Mukherjee et al., 1998).
B. Measurement of Recycling of NBDLabeled Sphingomyelin Using Dithionite
The dithionite method is a simple procedure used to bleach NBD located outside cells. With this procedure, it is possible to quantitate the appearance of NBD-lipid to the plasma membrane.
Solutions
The fluorescent lipid solution is prepared as described in Section III,A. Sodium dithionite solution is prepared by diluting a freshly made 1M aqueous stock solution with DMEM (final concentration: 50 mM).
Steps
- Grow HeLa cells in triplicate on 35-mm glassbottom culture dishes for 1-2 days to reach 50-80% confluency.
- Wash cells twice with ice-cold DMEM.
- Incubate cells for 30min at 4°C with 1 ml DMEM containing 4µM C6-NBD-SM.
- Wash cells twice with ice-cold DMEM and then add 1 ml prewarmed (37°C) DMEM.
- Incubate cells for 30min at 37°C in a CO2 incubator.
- Wash one dish twice with DMEM F-12 at room temperature and then add 1 ml DMEM F-12 and observe under a microscope (Fig. 3, "before quench").
- Wash the other two dishes twice with DMEM at room temperature.
- Add 1 ml DMEM containing 50mM sodium dithionite. Incubate for 1 min at room temperature.
- Wash one dish twice with DMEM F-12 at room temperature and then add 1 ml DMEM F-12 and observe under a microscope (Fig. 3, "after quench").
- Wash the last dish twice with DMEM at room temperature.
- Incubate cells in 1 ml DMEM for 30min at 37°C in a CO2 incubator.
- Wash cells twice with DMEM F-12 at room temperature.
- Add 1 ml DMEM F-12 and observe under a microscope (Fig. 3, "quench + 30min chase").
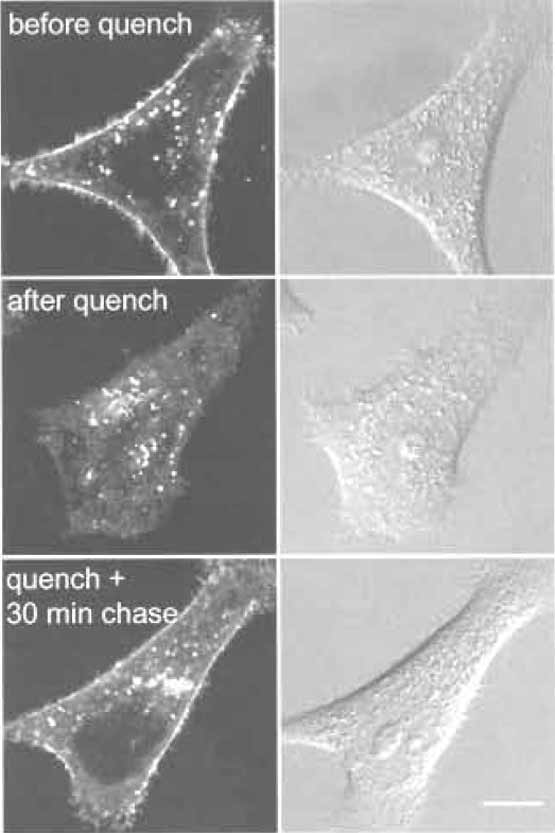 |
| FIGURE 3 HeLa cells labeled with C6-NBD-SM for 30min at 37°C (before quench). Cell surface fluorescent lipids are then quenched by sodium dithionite (after quench). Cells are further incubated for 30min at 37°C (quench + 30min chase). Bar: 10µm. |
Steps
- Grow HeLa cells in duplicate on 60-mm culture dishes for 1-2 days to reach 50-80% confluency.
- Wash cells twice with ice-cold DMEM.
- Incubate cells for 30min at 4°C with 2ml DMEM containing 4µM C6-NBD-SM.
- Wash cells twice with ice-cold DMEM and then add 2ml prewarmed (37°C) DMEM.
- Incubate cells for 30min at 37°C in a CO2 incubator.
- Wash cells twice with DMEM at room temperature.
- Add 2ml DMEM containing 50mM sodium dithionite. Incubate for 1 min at room temperature.
- Wash one dish twice with PBS at room temperature and then add 1 ml PBS containing 1% Triton X-100. Scrape cells and measure fluorescence at 535nm with excitation at 475nm using the Jasco FP-6500 spectrofluorometer (Fig. 4, "1st quench").
- Wash the other dish twice with DMEM at room temperature.
- Incubate cells in 2ml DMEM for 30min at 37°C in a CO2 incubator.
- Add 2ml DMEM containing 50mM sodium dithionite. Incubate for 1 min at room temperature.
- Wash cells twice with PBS at room temperature and then add 1 ml PBS containing 1% Triton X-100.
- Scrape cells and measure fluorescence at 535nm with excitation at 475 nm using the Jasco FP-6500 spectrofluorometer (Fig. 4, "2nd quench").
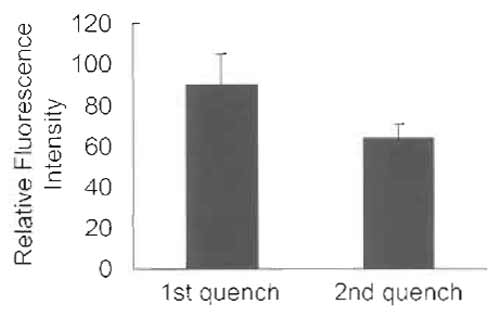 |
| FIGURE 4 HeLa cells labeled with C6-NBD-SM for 30min at 37°C and treated immediately with sodium dithionite (first quench) or incubated further for 30min at 37°C in DMEM after the first dithionite treatment. Cells are then again treated with dithionite (second quench). Cells are scraped in PBS containing 1% Triton X-100. Fluorescence intensity/dish is shown. Results are mean ± SD of three experiments. The difference between the two histograms represents the NBD-lipid recycled back to the plasma membrane. |
Selective removal of fluorescent lipid present on the outer leaflet of the plasma membrane allows the quantitative analysis of the kinetics of lipid transport to the plasma membrane. This is achieved by extracting C6-NBD- and C5-BODIPY-labeled phospho- and sphingolipids with BSA (Sleight and Pagano, 1984). Alternatively, one can selectively destroy NBD-labeled lipids on the outer monolayer with the water-soluble quencher dithionite (McIntyre and Sleight, 1991). The dithionite method is widely used to monitor transbilayer distribution (Nolan et al., 1995; Williamson et al., 1995) and vesicular traffic (Babia et al., 2001), as well as lateral mobility (Kobayashi et al., 1992), of NBDlabeled lipids. However, because dithionite can leak through the membrane in some cells, proper conditions have to be adapted to each specific case (Pomorski et al., 1994). Recycling of C6-NBD-SM is very rapid (Hao and Maxfield, 2000). The distribution of C6-NBD-SM shown here is the result of several recycling rounds of the fluorescent lipid. Internalized C6-NBD-SM is also transported to lysosomes and metabolized to C6-NBD-Cer. Transport to the degradative compartments is estimated to be 18- to 19-fold slower than the rate of C6-NBD-SM recycling (Koval and Pagano, 1990). In some cells, degradation of C6-NBD-SM occurs on the plasma membrane in a differentiation-dependent manner (Kok et al., 1995). Such degradation products are separated from C6- NBD-SM by thin-layer chromatography (Kobayashi and Pagano, 1989; Koval and Pagano, 1990).
C. Vital Stain of Golgi Apparatus by Fluorescent Ceramide Analogs
Fluorescent ceramide is widely used for vital staining of the Golgi apparatus (Pagano, 1989; Pagano et al., 1991). Dynamics of the Golgi membranes could be followed by using fluorescent ceramide.
1. Vital Stain of Golgi Apparatus by Fluorescent Ceramides
Stock solutions (1 mg/ml) of fluorescent lipids are prepared by dissolving C6-NBD-Cer, C12-NBD-Cer, and C5-BODIPY-Cer in ethanol. An aliquot of a stock lipid solution is dried, first under a stream of nitrogen and then in vacuo. The dried lipid is dissolved in DMEM to give a final concentration 0.5 µM (C6-NBDCer and C5-BODIPY-Cer) or 5 µM (C12-NBD-Cer). The solutions are vortexed and kept at 4°C before use.
Steps
- Grow HeLa cells on 35-mm glass-bottom culture dishes for 1-2 days to reach 50-80% confluency.
- Wash cells twice with DMEM at room temperature.
- Incubate cells for 30min at 37°C with 1ml DMEM containing 0.5 gM C6-NBD-Cer or C5-BODIPY-Cer or 5 gM C12-NBD-Cer in a CO2 incubator.
- Wash cells twice with DMEM F-12 at room temperature.
- Add 1 ml DMEM F-12 and observe under a microscope (Fig. 5.).
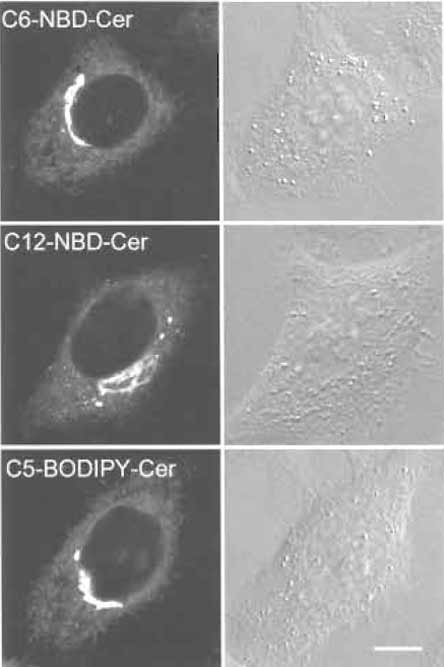 |
| FIGURE 5 HeLa cells labeled with fluorescent ceramide analogs. Bar: 10 µm. |
2. Alteration of Morphology of Golgi Apparatus Monitored by C5-BODIPY-Cer
Lipid solutions are prepared as described in Section III,C,1. 5-mg/ml stock solution of brefeldin A is diluted with DMEM F-12 to give a final concentration 5 µg/ml.
Steps
- Grow HeLa cells on 35-mm glass-bottom culture dishes for 1-2 days to reach 50-80% confluency.
- Wash cells twice with DMEM at room temperature.
- Incubate cells for 30min at 37°C with 1 ml DMEM containing 0.5 µM C5-BODIPY-Cer in a CO2 incubator.
- Wash cells twice with DMEM F-12 at room temperature.
- Add 1 ml DMEM F-12 containing 5µg/ml brefeldin A.
- Acquire images at appropriate intervals under a confocal microscope at room temperature (Fig. 6.).
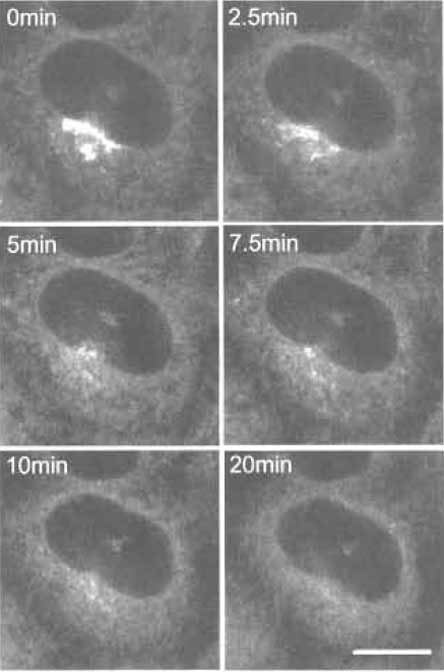 |
| FIGURE 6 C5-BODIPY-Cer-labeled HeLa cells treated with brefeldin A for various times. Bar: 10 µm |
Three different fluorescent ceramides are now available commercially. All three give similar Golgi labeling, although their working concentrations are different. Mechanisms of the accumulation of NBDlabeled ceramide and BODIPY-labeled ceramide are apparently different (Martin et al., 1993). Fluorescent ceramide makes it possible to follow the fate of Golgi lipids in living cells under various conditions such as brefeldin A treatment (Lippincott-Schwartz et al., 1991). Brefeldin A treatment induces redistribution of the Golgi apparatus into the endoplasmic reticulum (Chardin and McCormick, 1999). In Fig. 6, we adjusted the laser power of the confocal microscope to prevent bleaching of the fluorescence during observation. Spectral properties of BODIPY-labeled ceramides also allow us to differentiate membranes containing high concentrations of the fluorescent lipid and its metabolites from other regions of the cell where smaller amounts of the probe are present with a specific filter setup (Pagano et al., 1991).
IV. PITFALLS
- Because of the evaporation of solvent, the stock lipid solution is gradually concentrated during storage. Concentrations of NBD- and BODIPY-lipid solution are adjusted by measuring absorbance at 475 nm and comparing the OD with that of the solution of a known concentration.
- Degradation of lipids often occurs during storage. The purity of the lipid stock is checked periodically by thin-layer chromatography. Lipids are repurified or a new lot of fluorescent lipid is purchased when degradation compounds are detected.
Babia, T., Ledesma, M. D., Saffrich, R., Kok, J. W., Dotti, C. G., and Egea, G. (2001). Endocytosis of NBD-sphingolipids in neurons: exclusion from degradative compartments and transport to the Golgi complex. Traffic 2, 395-405.
Chardin, P., and McCormick, E (1999). Brefeldin A: The advantage of being uncompetitive. Cell 97, 153-155.
Devaux, P. E, Fellmann, P., and Herve, P. (2002). Investigation on lipid asymmetry using lipid probes: Comparison between spin-labeled lipids and fluorescent lipids. Chem. Phys. Lipids 116, 115-134.
HaG, M., and Maxfield, E R. (2000). Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 275, 15279-15286.
Kobayashi, T., and Pagano, R. E. (1989). Lipid transport during mitosis: Alternative pathways for delivery of newly synthesized lipids to the cell surface. J. Biol. Chem 264, 5966-5973.
Kobayashi, T., Storrie, B., Simons, K., and Dotti, C. G. (1992). A functional barrier to movement of lipids in polarized neurons. Nature 359, 647-650.
Kok, J. W., Babia, T., Klappe, K., and Hoekstra, D. (1995). Fluorescent, short-chain C6-NBD-sphingomyelin, but not C6-NBDglucosylceramide, is subject to extensive degradation in the plasma membrane: Implications for signal transduction related to cell differentiation. Biochem. J. 309, 905-912.
Koval, M., and Pagano, R. E. (1990). Sorting of an internalized plasma membrane lipid between recycling and degradative pathways in normal and Niemann-Pick, type A fibroblasts. J. Cell Biol. 111, 429-442.
Lippincott-Schwartz, J., Glickman, J., Donaldson, J. G., Robbins, J., Kreis, T. E., Seamon, K. B., Sheetz, M. P., and Klausner, R. D. (1991). Forskolin inhibits and reverses the effects of brefeldin A on Golgi morphology by a cAMP-independent mechanism. J. Cell Biol. 112, 567-577.
Maier, O., Oberle, V., and Hoekstra, D. (2002). Fluorescent lipid probes: Some properties and applications. Chem. Phys. Lipids 116, 3-18.
Martin, O. C., Comly, M. E., Blanchette-Mackie, E. J., Pentchev, P. G., and Pagano, R. E. (1993). Cholesterol deprivation affects the fluorescence properties of a ceramide analog at the Golgi apparatus of living cells. Proc. Natl. Acad. Sci. USA 90, 2661-2665.
Martin, O. C., and Pagano, R. E. (1987). Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells: Evidence for a protein-mediated and ATP-dependent process(es). J. Biol. Chem. 262, 5890-5898.
Mayor, S., Presley, J. E, and Maxfield, E R. (1993). Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 121, 1257-1269.
Mukherjee, S., Zha, X., Tabas, I., and Maxfield, E R. (1998). Cholesterol distribution in living cells: Fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys. J. 75, 1915-1925.
Nolan, J. P., Magargee, S. E, Posner, R. G., and Hammerstedt, R. H. (1995). Flow cytometric analysis of transmembrane phospholipid movement in bull sperm. Biochemistry 34, 3907-3915.
Pagano, R. E. (1989). A fluorescent derivative of ceramide: Physical properties and use in studying the Golgi apparatus of animal cells. Method Cell Biol. 29, 75-85.
Pagano, R. E., and Chen, C. S. (1998). Use of BODIPY-labeled sphingolipids to study membrane traffic along the endocytic pathway. Ann. N. Y. Acad. Sci. 845, 152-160.
Pagano, R. E., Martin, O. C., Kang, H. C., and Haugland, R. P. (1991). A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: Accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J. Cell Biol. 113, 1267-1279.
Sleight, R. G., and Pagano, R. E. (1984). Transport of a fluorescent phosphatidylcholine analog from the plasma membrane to the Golgi apparatus. J. Cell Biol. 99, 742-751.
Somerharju, P. (2002). Pyrene-labeled lipids as tools in membrane biophysics and cell biology. Chem. Phys. Lipids 116, 57-74.
Williamson, P., Bevers, E. M., Smeets, E. E, Comfurius, P., Schlegel, R. A., and Zwaal, R. E (1995). Continuous analysis of the mechanism of activated transbilayer lipid movement in platelets. Biochemistry 34, 10448-10455.




