Empirical and Structural Formulas
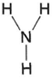 |
| Figure 4-1 Structural formula for ammonia |
| Figure 4-2 Structural formula for carbon dioxide |
Organic chemistry can be simply defined as the chemistry of carbon compounds. Inorganic chemistry, then, is all the rest. In inorganic chemistry, empirical formulas for compounds utilize the appropriate atomic symbols. For example, ammonia has one atom of nitrogen and three atoms of hydrogen in its molecule. Its empirical formula, therefore, is NH3,. In organic chemistry, however, structural formulas are necessary. In organic chemistry, ammonia (an inorganic compound) would thus be represented as in figure 4-1. The lines represent single bonds. The structural formula for carbon dioxide, CO2, is shown in figure 4-2. Figure 4-2 shows that the oxygen atoms in carbon dioxide are attached to the carbon atoms. Further, two bonds are involved in the linkage. We may say, then, that oxygen has two bonds and carbon has four bonds.
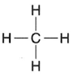 |
| Figure 4-3 Structural formula for methane. |
Now consider the structural formula for methane (figure 4-3).
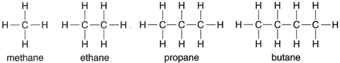 |
| Figure 4-4 Structural formulas for methane, ethane, propane, and biane. |
This formula shows that the hydrogen atoms are attached to the carbon atoms, a fact that would not be evident if the empirical formula CH4 were used. Such is the case with all compounds. Using methane as a
starter, a whole series of compounds can be represented, as shown in figure 4-4.
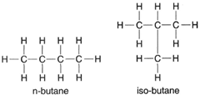 |
| Figure 4-5 Structural formulas for n-butane and iso-butane. |
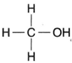 |
| Figure 4-6 Structural formula for methyl alcohol |
The 4-carbon compound butane furthesrh ows the necessity of structural formulas. Both compounds shown in figure 4-5 can be represented by the empirical formula C4H10. They are, however, quite different compounds. The first is normal butane (n-butane), and the second is iso-butane, a substance with significantly different properties.




