Hydrogenation
The production of ethyl alcohol by one pathway and acetic acid by the other
cannot be attributed solely to one process being anaerobic and the other
being aerobic. These chemical changes are controlled by enzymes, and the
kinds of reactions that occur depend on the kinds of enzymes available. One
type of reaction that occurs as carbohydrate is broken down is called
dehydrogenation (wherein hydrogen atoms are removed from the molecule). The
particular enzyme required for dehydrogenation is called
dehydrogenase.
When the hydrogen bonds are broken, energy is released. Some of this energy
is used to maintain the reaction, some is lost as heat, and some is used in
the formation of ATP The removal of hydrogen atoms requires a hydrogen
acceptor. In aerobic respiration, the hydrogen acceptor is oxygen. Hydrogenation
in aerobic respiration, then, results in the manufacture of water. This
is not a one step process; rather, it is accomplished by a series of transfers
from carrier to carrier (figure 10-8). As the hydrogen passes from one carrier
to the next, energy is released at each step (figure 10-9).
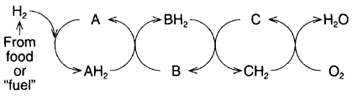 |
| Figure 10-8 Hydrogen obtained from "food" passes through several different steps
before contributing to the formation of water. |
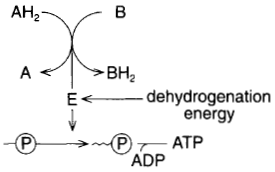 |
| Figure 10-9 An elaboration of figure 10-8. As hydrogen is passed from one molecule to
another, energy is released and used in the manufacture of ATP.. |






