G Proteins
G proteins are important intermediates in signal transduction pathways because they determine whether the signal will be stimulatory or inhibitory. Amajor family of G proteins is trimeric, consisting of three subunits: α, β, and γ. The α subunit is capable of binding GDPor GTP. When a signal stimulates the receptor, the altered receptor stimulates a change in the G protein: GDP dissociates from the α subunit, and GTP takes its place. This stimulates dissociation of the α subunit, which then diffuses along the inner surface of the membrane until it contacts an enzyme or ion pore. The activity of the α subunit is blocked when the bound GTP is hydrolyzed and it reassociates with the β and γ subunits. Trimeric G proteins affect ion pores and enzymes such as adenylcyclases, guanylcyclases, and phospholipases. The pores may be opened or closed, and the enzymes may be stimulated or inhibited. These enzymes are important in signal pathways because they amplify weak signals by catalytically producing second messengers.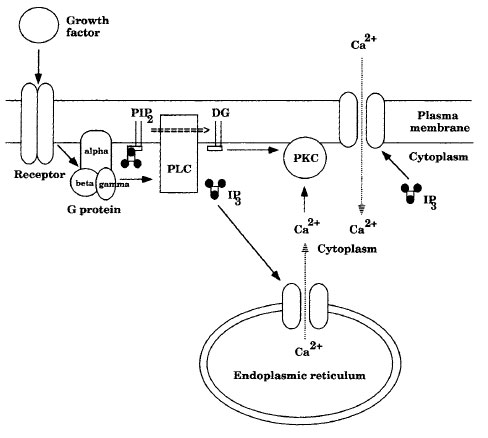 |
| Figure 11-1 A signal transduction pathway. |
A signal transduction pathway involving IP3 and DG is shown in Figure 11-1. Agrowth factor or hormone binding to a cell-membrane receptor alters the receptor's conformation, which stimulates the dissociation of a neighboring trimeric G protein and a GDP attached to the α subunit of the G protein. The α subunit becomes active in the signal transduction pathway by dissociating from the β and γ subunits of the G protein and exchanging a molecule of GTP for GDP. The active G protein stimulates a membrane bound phospholipase C (PLC) that hydrolyzes the phosphatidylinositol 4',5'-bisphosphate (PIP2) in the membrane to DG and inositol 1',4',5'-triphosphate (IP3). IP3 binding to calcium ion pores opens these pores in the ER and the plasma membrane, allowing calcium ions to move along their concentration gradient from the ER and from the extracellular environment into the cytoplasm. Calcium ions and DG binding to inactive protein kinase C (PKC) causes PKC to become active. Activated PKC phosphorylates other protein kinases in signal transduction pathways, often activating them.
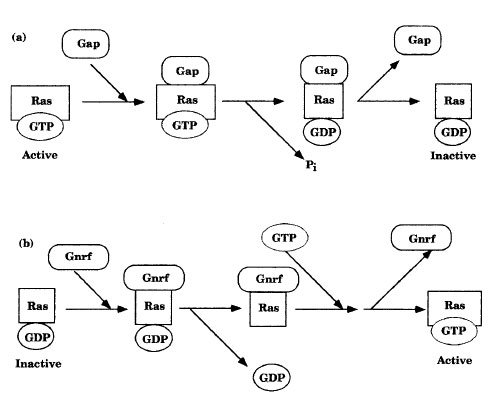 |
| Figure 11-2 Regulation of the ras gene product by (a) GAPs and (b) GNRFs. |
A second family of G proteins consist of a single subunit. These monomeric proteins are known as Ras proteins and are activated indirectly through autophosphorylation of membrane-bound tyrosine kinases and the regulatory proteins that interact with the phosphates (Figure 11-2). The relative amounts of active and inactive Ras are determined by guanine nucleotide release factors (GNRFs) and by GTPase-activating proteins (GAPs). Since these proteins promote the exchange of GTP for GDP, or GDP for GTP, respectfully, they affect Ras protein activity. Hydrolysis of GTP to GDP and Pi inhibits Ras. Some Ras proteins are negatively regulated by tumor suppressor proteins.
Notes Ras proteins generally stimulate a cascade of protein kinases, whereas trimeric G proteins usually inhibit or stimulate enzymes such as adenylcyclase.




